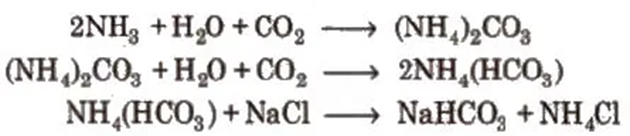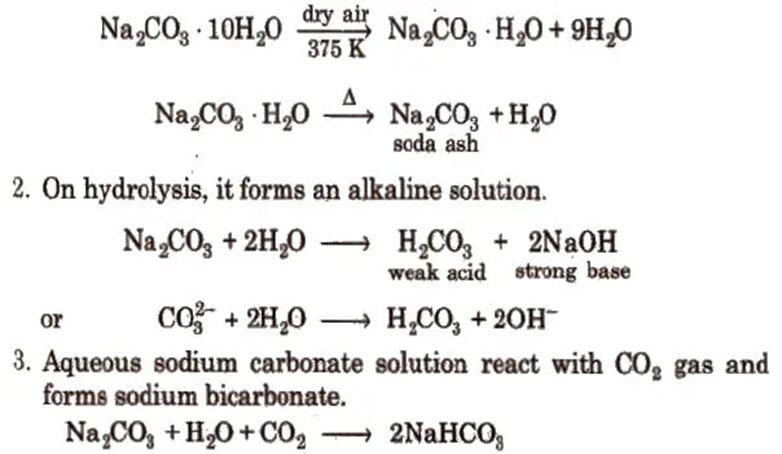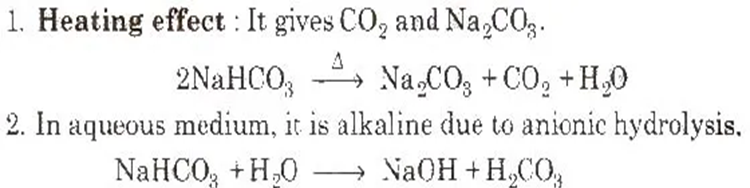1. CLASSICAL IDEA OF REDOX REACTIONS
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
CHAPTER - 8 REDOX REACTIONS
CLASSICAL IDEA OF REDOX REACTIONS – OXIDATION AND REDUCTION REACTIONS
• Oxidation
Oxidation is defined as the addition of oxygen/electronegative element to a substance or rememoval of hydrogen/ electropositive element from a susbtance.
For example,
2Mg(s) + O2(g) → 2MgO(s)
Mg(s) + Cl2(g) → 2MgCl2
• Reduction
Reduction is defined as the memoval of oxygen/electronegative element from a substance or addition of hydrogen or electropositive element to a substance.
For example,
2FeCl3(aq) + H2(g) → 2FeCl2(aq) + 2HCl(aq)
2HgO(s) → H2(g) → 2Hg(l) + O2(g)
2. REDOX REACTIONS IN TERMS OF ELECTRON TRANSFER REACTIONS
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
REDOX REACTION IN TERMS OF ELECTRON TRANSFER REACTION
A few examples of redox reaction on the basis of electronic concept are given below:
According to electronic concept every redox reaction consists of two steps known as half reactions.
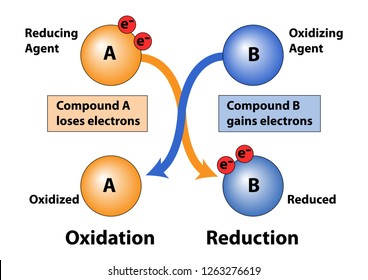
(i) Oxidation reaction: Half reactions that involve loss of electrons are called oxidation reactions.
(ii) Reduction reaction: Half reactions that involve gain of electrons are called reduction reactions.
Oxidising agent: Acceptor of electrons.
Reducing agent: Donar of electrons.
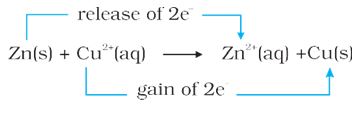
Zn + Cu2+ → Zn2+ + Cu
• Competitive Electron Transfer Reactions
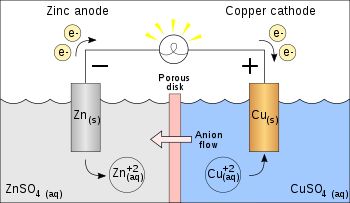
To understand this concept let us do an experiment.
Place a strip of metallic zinc in an aqueous solution of copper nitrate as shown in Fig. After one hour following changes will be noticed.
(i) Strips becomes coated with reddish metallic copper.
(ii) Blue colour of the solution disappears.
(iii) If hydrogen sulphide gas is passed through the solution appearance of white ZnS can be – seen on making the solution alkaline with ammonia.
3. OXIDATION NUMBER
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
OXIDATION NUMBER
It is the oxidation state of an element in a compound which is the charge assigned to an atom of a compound is equal to the number of electrons in the valence shell of an atom that are gained or lost completely or to a large extent by that atom while forming a bond in a compound.
• Rules for Assigning Oxidation Numbers
(i) The oxidation number of an element in its elementary form is zero. For example, H2, 02, N2 etc. have oxidation number equal to zero.
(ii)In a single monoatomic ion, the oxidation number is equal to the charge on the ion. For example, Na+ ion has oxidation number of +1 and Mg2+ ion has +2.
(iii) Oxygen has oxidation number -2 in its compounds. However, there are some exceptions.
Compounds such as peroxides. Na202, H202
oxidation number of oxygen = – 1 In OF2
O.N. of oxygen = +2 02F2
O.N. of oxygen = +1
(iv) In non-metallic compounds of hydrogen like HCl, H2S, H2O oxidation number of hydrogen = + 1 but in metal hydrides oxidation number of hydrogen = -1
[LiH, NaH, CaH2 etc.]
(v) In compounds of metals and non-metals metals have positive oxidation number while non-metals have negative oxidation number. For example, In NaCl. Na has +1 oxidation number while chlorine has -1.
(vi) If in a compound there are two non-metallic atoms the atoms with high electronegativity is assigned negative oxidation number while other atoms have positive oxidation number.
(vii) The algebraic sum of the oxidation number of all atoms in a compound is equal to zero.
(viii) In poly atomic ion the sum of the oxidation no. of all the atoms in the ion is equal to the net charge on the ion. For example, in (C03)2—Sum of carbon atoms and three oxygen atoms is equal to -2.
Fluorine (F2) is so highly reactive non-metal that it displaces oxygen from water.
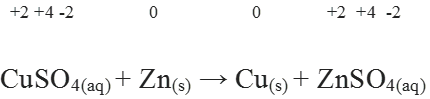
Disproportionation Reaction. In a disproportionation reaction an element in one oxidation state is simultaneously oxidises and reduced.
For example,
Hence, the oxygen of peroxide, which is present in -1 oxidation state is connected to zero oxidation state and in 02 and in H2O decreases to -2 oxidation state.
• Fractional Oxidation Numbers
Elements as such do not have any fractional oxidation numbers. When the same element are involved in different bonding in a species, their actual oxidation states are whole numbers but an average of these is fractional.
For example, In C302
![]()
Fractional O.N. of a particular element can be claculated only if we know about the structure of the compound or in which it is present.
• Balancing of Redox Reactions
(i) Oxidation Number Method. Following steps are involved:
(ii) Write the correct formula for each reactant and product.
(b) By assigning the oxidation change in oxidation number can be identified.
(c) Calculate the increase and decrease in oxidation number per atom with respect to the reactants. If more than one atom is present then multiply by suitable coefficient.
(d) Balance the equation with respect to all atoms. Balance hydrogen and oxygen atoms also.
(e) If the reaction is carried out in acidic medium, use H+ ions in the equation. If it is in basic medium use OH– ions.
(f) Hydrogen atoms in the expression can be balanced by adding (H20) molecules to the reactants or products.
If there are the same number of oxygen atoms on the both side of equation then it represents the balanced redox reaction.
(ii) Half Reaction Method. In this method two half equation are balanced separately and than added together to give balanced equation.
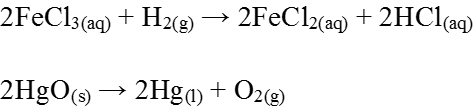
• Redox Reactions as the Basis for Titration
Potassium Permanganate Titration: In these titrations potassium permanganate (pink in colour) acts as an oxidising agent in the acidic medium while oxalic acid or some ferrous salts acts as a reducing agents.
The ionic equation can be written as:
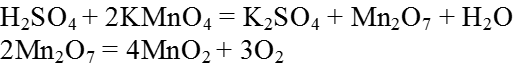
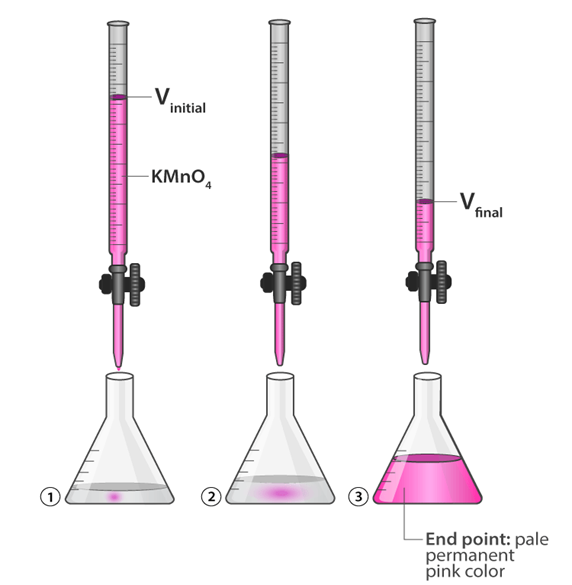
These are the examples of redox titration.
On both these titrations, potassium permanganate itself acts as indicator. It is commonly known as self indicator. The appearance of pink colour in the solution represents the end points.
Potassium Dichromate Titration: In place of potassium permanganate, potassium dichromate can also be used in the presence of dil. H2S04. The ionic equation for the redox reaction with FeS04 (Fe2+ ions) is given.
• Limitation of Concept of Oxidation Number
According to the concept of oxidation number, oxidation means increase in oxidation number – by loss of electrons and reduction means decrease in oxidation number by the gain of electrons. However, during oxidation there is decrease in electron density while increase in electron density around the atom undergoing reduction.
4. REDOX REACTIONS AND ELECTRODE PROCESSES
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
REDOX REACTIONS AND ELECTRODE PROCESSES—ELECTROCHEMICAL CELLS
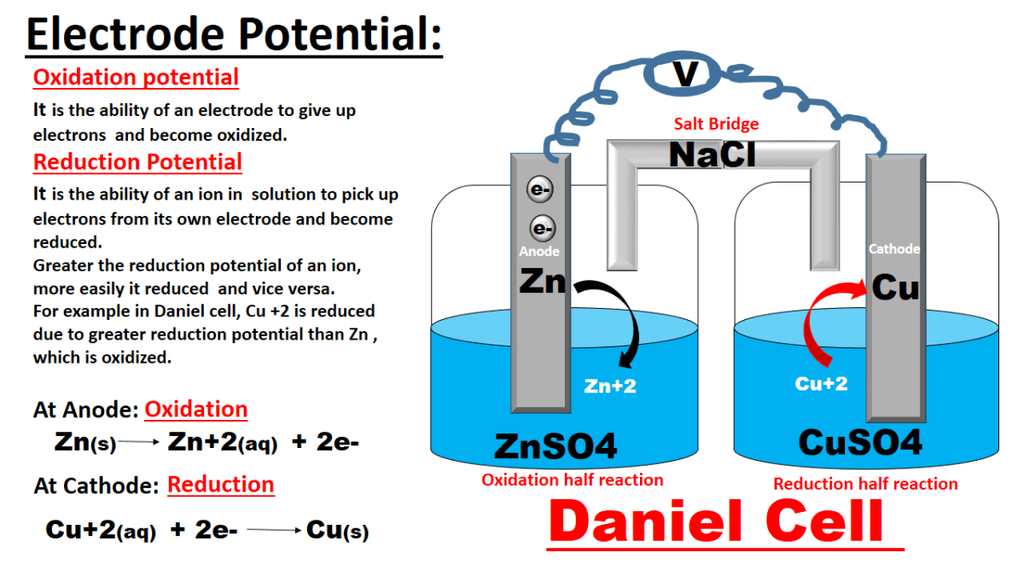
A device in which the redox reaction is carried indirectly and the decrease in energy appears as the electrical energy are called electrochemical cell.
Electrolytic Cell. The cell in which electrical energy is converted into chemical energy. Example, when lead storage battery is recharged, it acts as electrolytic cell.
Redox Reactions and Electrode Processes. When zinc rod is dipped in copper sulphate solution redox reaction begins hence, zinc is oxidised to Zn2+ ions and Cu2+ ions are reduced to metal.
●Types of Electrochemical Cell
Electrochemical cells are primarily of two types:
1.Galvanic cell or voltaic cell
2.Electrolytic cell
Let us learn about both of their key features and differences in tabular form:
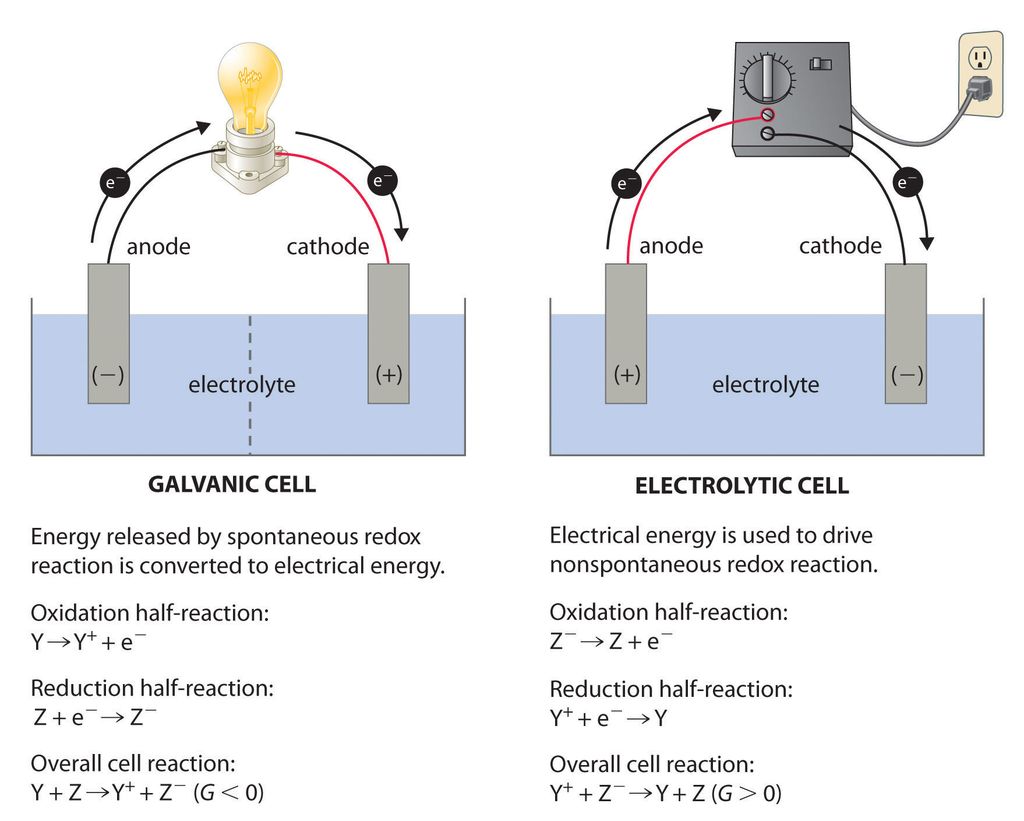
• Redox reaction. Reactions in which oxidation and reduction occur simultaneously are called redox reactions.
• Oxidation. Involves loss of one or more electrons.
• Reduction. Involves gain of one or more electrons.
• Oxidising agent. Accepting electrons.
• Reducing agent. Losing electrons.
• Electrochemical cell. It is a device in which redox reaction is carried indirectly and decrease in energy gives electrical energy.
• Electrode potential. It is the potential difference between the electrode and its ions in solution.
• Standard electrode potential. It is the potential of an electrode with respect to standard hydrogen electrode.
• Electrochemical series. It is activity series. It has been formed by arranging the metals in order of increasing standard reduction potential value.
1. Position of hydrogen in the perioidic table
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
CHAPTER - 9 HYDROGEN
POSITION OF HYDROGEN IN THE PERIODIC TABLE
• Electronic Configuration of Hydrogen 1s1
Position of hydrogen in the periodic table: Position of hydrogen in periodic table is not justified because it resembles both alkali metals as well as halogens.
• Resemblance of Hydrogen with Alkali Metals
(i) Electronic Configuration: Hydrogen has one electron in its valence shell like alkali metals.
H → H+ + e-
Na → Na+ + e-
(ii) Both hydrogen and alkali metals form unipositive ions.
For example,
Na → Na+ + e–
H → H+ + e–
(iii) Hydrogen and alkali metals both shows +1 oxidation state.
(iv) Hydrogen as well as other alkali metals acts as reducing agents.
(v) Both have affinity for electronegative element For example, Na2O, NaCl, H20, HCl.
• Resemblance with Hologens
(i) Electronic configuration: Hydrogen and halogen family both require one electron to fulfil the inert gas configuration
H-1s1 ; He-1s2
X-ns2np5 : Ne-1s22s22p6
(ii) Ionisation energy of hydrogen is almost similar to halogens.
(iii) Hydrogen as well as halogens are Diatomic in nature.
(iv) Many compounds of hydrogen as well as of halogens are of covalent nature.
For example, CH4, SiH4CCl4, SiCl4
1. Group 13 elements : The boron family
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
CHAPTER- 11 THE p BLOCK ELEMENTS
• p-Block Elements
Elements belonging to groups 13 to 18 of the periodic table are called p-block elements. General electronic configuration: ns2 np1-6 (except for He)
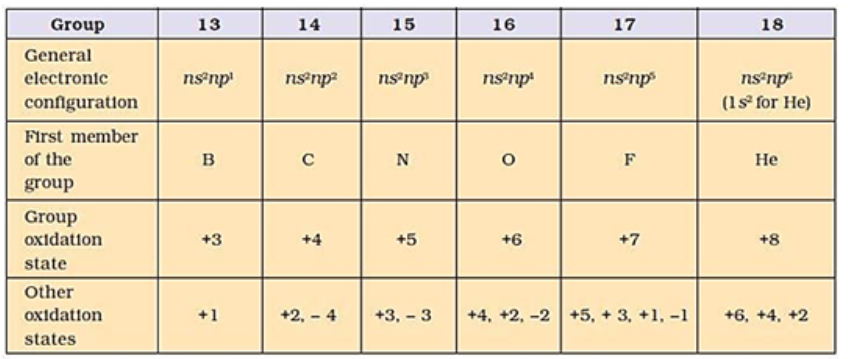
GROUP 13 ELEMENTS: THE BORON FAMILY
Outer Electronic Configuration: ns2np1
Atomic Radii: The atomic and ionic radii of group 13 elements are smaller than the corresponding elements of alkali and alkaline earth metals.
Reason: On moving from left to right in a period the effective nuclear charge increases and the outer electrons are pulled more strongly towards the nucleus. This results in decrease in atomic size.
On moving down the group, both atomic and ionic radii expected to increase due to the addition of a new electron shell with each succeeding element.
Exception: Atomic radius of Ga is less than that of Al due to the presence of poor shedding 10d-electrons in gallium.
Ionisation enthalpies: First ionisation enthalpies of the elements of group-13 are less than those of the elements present in group-2 in the same period.
Reason: The removal of p-electron is much easier than the s-electron and therefore, the first ionisation enthalpies (∆i H1) of the elements of group 13 are lower as compared to the corresponding elements of group 2.
On moving down the group 13 from B to Al the first-ionization enthalpies (∆i H1) decrease due to an increase in atomic size and screening effect which outweigh the effect of increased
nuclear charge.
There is discontinuity expected in the ionisation enthalpy values between Al and Ga and between In and Tl due to inability of d- and f-electrons which have low screening effect to compensate the increase in nuclear charge.
Electronegativity: Down the group, electronegativity first decreases from B to Al and then increases.
This is due to discrepancies in the atomic size of the elements.
Physical Properties
(i) Due to strong crystalline lattice boron has high melting point. Rest of the members of this family have low melting point.
(ii) Boron is extremely hard and black coloured solid and non metallic in nature.
(iii) Other members of this family are soft metals with low melting point and high electrical conductivity.
Chemical Properties
Oxidation states: The first two elements boron and aluminium show only +3 oxidation state ~ in the compounds but the other elements of this group gallium, indium and thalium also exhibit +1 oxidation state in addition to +3 oxidation state i.e., they show variable oxidation states.
As we move down the group, the stability of +3 oxidation state decreases while that of +1 oxidation state progressively increases.
1. General Introduction
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
CHAPTER- 12 ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES AND TECHNIQUES
GENERAL INTRODUCTION
Organic chemistry is the branch of chemistry that deals with carbon compounds. But all carbon compounds are not considered as organic compounds. (E.g. CO2, CO, metal carbonates, bicarbonates etc.). So organic chemistry can be defined as the branch of chemistry that deals with hydrocarbons and their derivatives. Hydrocarbons are the major class of organic compounds and they contain only carbon and hydrogen atoms. All other organic compounds are formed by replacing one or more hydrogen atoms of hydrocarbons by other atoms or groups (They are called hydrocarbon derivatives).
All carbon compounds present in plants and animals are organic compounds. E.g. Carbohydrates, proteins, vitamins, nucleic acids, amino acids, fats and oils, natural polymers etc. petroleum and coal are the major source of organic compounds (hydrocarbons).
In ancient times, it was believed that a vital force (living body) is necessary for the production of an organic compound. But in 1828, Frederic Wohler proved that this belief was wrong. He prepared urea in the laboratory, by heating ammonium cyanate (NH4CNO). It was the first organic compound prepared in the laboratory.
NH4CNO ⎯⎯ Heat⎯→ NH2CONH2
Then another scientist Kolbe synthesized acetic acid and Berthelot synthesized methane in the laboratory. Nowadays about 95% of the organic compounds are synthesized in the laboratory.
2. Dihydrogen
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
DIHYDROGEN, H2
• Occurrence of Hydrogen
Hydrogen is the most abundant element in the universe. It is present in combined state as water, coal, animal and vegetable matter. All organic compounds contain hydrogen as an essential constituent.
• Isotopes of Hydrogen
Hydrogen has three isotopes.
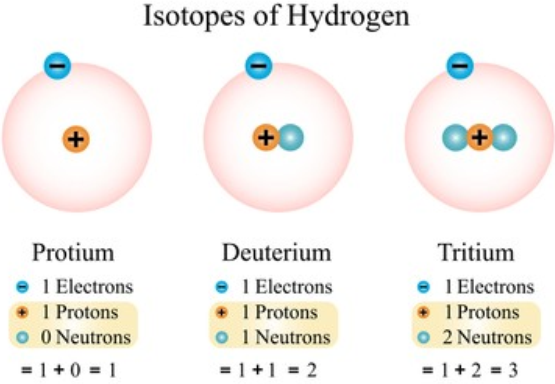
3. Preparation of dihydrogen
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
PREPARATION OF DIHYDROGEN, H2
Laboratory Preparation of Dihydrogen
(i) It is prepared by the reaction of granulated zinc with dil HCl.
Zn + 2HCl ——–> ZnCl2 + H2
(ii) It is prepared by the action of zinc with aqueous alkali.
2NaOH + Zn → Na2ZnO2 + H2
4. Properties of dihydrogen
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
PROPERTIES OF DIHYDROGEN
Physical properties
(i) Dihydrogen is a colourless, odourless and tasteless gas.
(ii) It is a combustible gas.
(iii) It is insoluble in water.
(iv) It is lighter than air.
Chemical properties
Reaction with halogens: It reacts with halogens, X2 to give hydrogen halides. HX.
H2 + X2 → 2HX (X= F,Cl,Br,I)
5. Hydrides
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
HYDRIDES
The hydrides are classified into three types:
(i) Ionic or saline or salt like hydrides
(ii) Covalent or molecular hydrides (iii) Metallic or non-stoichiometric hydrides.
• Ionic or Saline Hydrides
Hydrides formed between hydrogen and electropositive element of group I and II belonging to s-block. These are known as stoichiometric compounds.
Properties of saline or ionic hydrides:
(i) The hydrides of lighter elements like Li, Be, Mg etc. have significant covalent character.
(ii) Ionic hydrides are crystalline, non-volatile and non-conducting in solid state.
(iii) They conduct electricity in molten state and liberate hydrogen at anode.
• Covalent or Molecular Hydrides
These are binary compounds of hydrogen with non-metals belonging to p-block.
For example, NH3, CH4, H20, HF They are mostly volatile compounds with low boiling points. They are classified as:
(i) Electron-Deficient Molecular Hydride: Molecular hydrides in which central atom does not have octet are called electron deficient hydrides e.g., BH3, MgH2, BeH2.
(ii) Electron precise hydrides: Those hydrides in which the central atom has its octet complete e.g., group 14 hydrides. They are tetrahedral in geometry.
(iii) Electron rich hydrides: Those metal hydrides which contain lone pair of electrons are called electron rich hydrides, e.g., NH3, PH3, H20 and H2S.
NH3 and PH3 has 1 lone pair and H20 and H2S have 2 lone pairs of electrons.
• Metallic or Non-Stoichiometric Hydrides
These hydrides are also known as interstitial hydrides. Transition metals group 3, 4 and 5 form metallic hydrides. In group 6, chromium alone has a tendency to form CrH. Metals of 7, 8 and 9 do not form hydrides. This is called as hydride gap.
Latest study shows that only Ni, Pd, Ce and Ac are interstitial in nature, that means they can occupy hydrogen atom in the interstitial sides. The hydrides are generally non-stoichiometric and their composition varies with temperature and pressure, for example, Ti H1.73, CeH2.7′ , LaH2.8 etc.
These hydrides have metallic lock and their properties are closely related to those of the parent metal. They are strong reducing agents in most of the cases due to the presence of free hydrogen atom in the metal lattice.
6. Water
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
WATER
Human body has about 65% and some plants have nearly 95% water.
Physical properties of water:
(i) Freezing point of water is 273.15 K and boiling point 373.15 K.
(ii) Maximum density of water at 4°C is 1 gm cm-3
(iii) It is a colourless and tasteless liquid.
(iv) Due to hydrogen bonding with polar molecules, even covalent compounds like alcohol and carbohydrates dissolve in water.
Structure of Water:
In gas phase, it is a bent molecule with HOH bond angle 104.5° and O—H bond length of 95.7 pm. It is highly polar in nature. Its orbital overlap picture is also shown below.
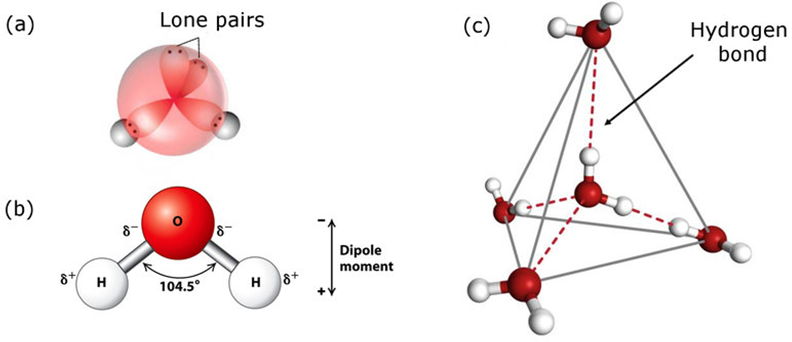
Water in Crystalline Form:
Ice is the crystalline form of water. At atmospheric pressure ice crystallise in the hexagonal form. At low temperature it condenses to cubic form. Density of ice is less than that of water. Therefore, ice cubes can float on water.
Structure of ice:
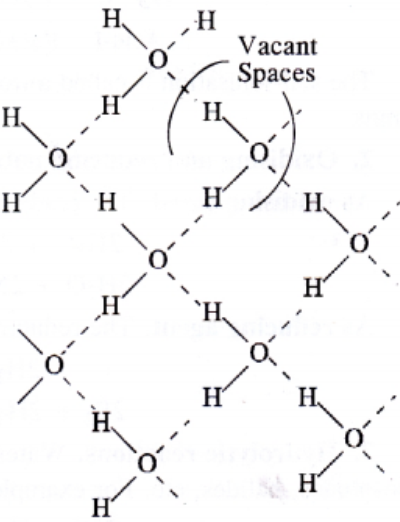
Chemical Properties of Water:
(i) Amphoteric nature: It behaves like an amphoteric substance because it can act as an acid as well as base.
H2O + HCl → H3O+ + Cl-
Autoprotolysis of water also accounts for its amphoteric nature according to Bronsted-Lowry concept.
H2O(l) + H2O(l) ↔ H3O+(aq) + OH-(aq)
pH of water is 7 and it is neutral towards pH.
(ii) Oxidising and Reducing Nature: Water can act as an oxidising as well as reducing agent
(iii) Hydrolysis Reaction: It has a very strong hydrating tendency. It can hydrolyse a large number of compounds such as oxides, halides, carbides etc.

• Hydrates Formation
From aqueous solutions many salts can be crystallised as hydrated salts. Hydrates are of three types:
(i) Coordinated water
For example: [Ni(H20)6]2+ (N03–)2 and [Cr(H20)6]3+ 3CP
(ii) Interstitial water
For example: BaCl2. 2H20
(iii) Hydrogen bonded water
For example: [Cu(H20)4]2+ S042- H20 in CuS04.5H2
• Hard and Soft Water
Hard water: Water which does not produce lather with soap easily is called hard water. Presence of calcium and magnesium salts in the form of hydrogen carbonate, chloride and sulphate in water makes the water hard.
Types of Hardness of Water:
(i) Temporary hardness: It is due to the presence of bicarbonates of calcium and magnesium in water. It is known as temporary because it can be easily removed by simple boiling of hard water.
(ii) Permanent hardness: It is due to the presence of chlorides and sulphates of calcium and magnesium. It cannot be removed on boiling water. Permanent hardness of water can be removed by chemical methods.
Soft water: Water which readily forms lather with soap is called soft water.
For example: rain water, distilled water.
7. Hydrogen peroxide
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
HYDROGEN PEROXIDE (H202)
Preparation: Merck's method
Sodium peroxide is gradually added to an ice-cold solution of 20% H2SO4.
Na2O2+H2SO4→Na2SO4+H2O2
Upon cooling, crystals of Na2SO4.10H2O separates out and the resulting solution contains 30% H2O2.
Preparation of hydrogen peroxide from barium peroxide
By the action of dilute sulphuric acid:
BaO2.8H2O(s)+H2SO4(aq)→BaSO4(s)+H2O2(aq)+8H2O(l)
By the action of carbon dioxide:
BaO2+H2O+CO2→BaCO3+H2O2
By the action of sulphuric acid:
3BaO2+2H3PO4→Ba3(PO4)2+3H2O2
Limitation of preparation of hydrogen peroxide
H2O2 prepared from barium peroxide ( laboratory method of preparation ) contains appreciable amount of Ba2+ ions which catalyse the decomposition of H2O2. Therefore, hydrogen peroxide cannot be stored for a long time. It is unstable and also explosive, so it is usually preserved in water solution.
Manufacture of hydrogen peroxide
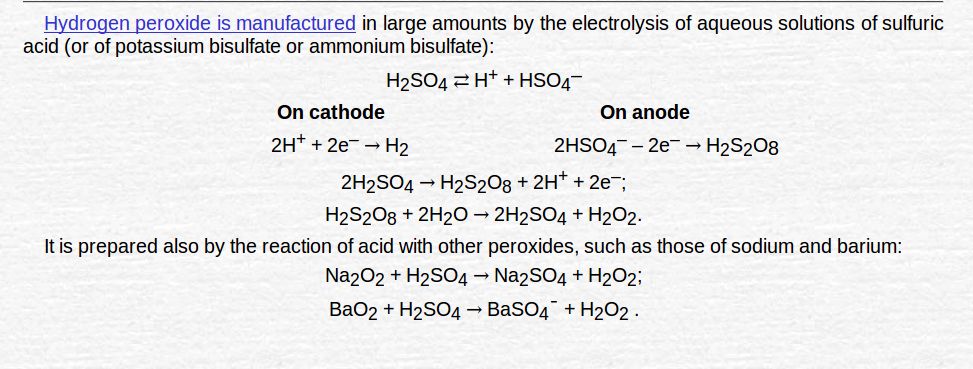
- By electrolysis of 50% H2O2
- By auto oxidation of 2-ethylanthraquinol
Methods of concentration of Hydrogen peroxide
- Evaporation on a water bath
- Dehydration in a vacuum dessicator
- Distillation under reduced pressure
- Removal of last traces of water
Strength of hydrogen peroxide solution
Strength is expressed in two ways:
- Percentage strength: It expresses the amount of H2O2 by weight present in 100 ml of the solution.
- Volume strength: The volume of oxygen liberated at N.T.P by the decomposition of 1 ml of that sample of hydrogen peroxide.
Physical properties of Hydrogen peroxide
Hydrogen peroxide is colorless and odorless liquid.
2) It is bitter in taste.
3) Pure H2O2 is thick syrupy liquid with pale blue colour.
4) It is completely miscible with water, alcohol and ether in all proportion.
Chemical properties of hydrogen peroxide
1. Decomposition: Pure H2O2 is unstable in nature, hence it decomposes into water and oxygen.
2H2O2(aq)→2H2O(l)+O2(g)
2. Acidic nature: It turns blue litmus red but it dil. solution is neutral to litmus. The acidic nature of H2O2 is shown by its neutralization reactions with hydroxides.
NaOH+H2O2→NaHO2+H2O
Ba(OH)2+H2O→BaO2+2H2O
3. Oxidising and reducing nature:
It oxidises lead sulphide to lead sulphate (in neutral solution)
It oxidizes acidified ferrous sulphate to ferric sulphate (in acidic medium)
H2O2→H2O+O
2FeSO4+H2SO4+H2O2→Fe2(SO4)3+H2O
4. In the presence of other oxidizing agents, hydrogen peroxide acts as a reducing agent. This is because it can take up an atom of oxygen to give water and oxygen gas.
Ag2O+H2O2→2Ag+H2O+O2
Tests of hydrogen peroxide
- It liberates iodine from KI solution.
- It decolourizes KMnO4 solution.
- It turns filter paper containing black stains of PbS white.
Structure of hydrogen peroxide
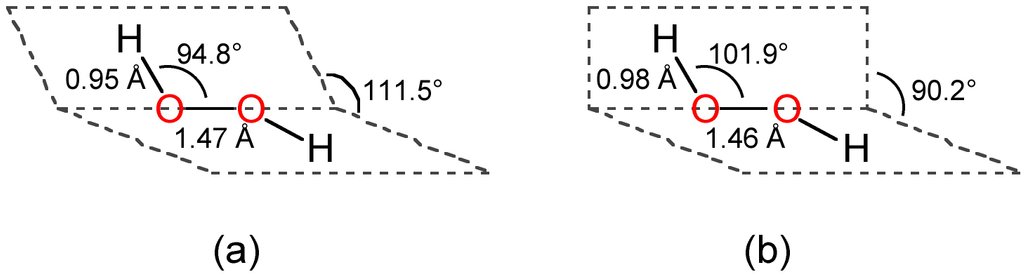
It has a open book structure. It is shown in the image.
Uses of hydrogen peroxide
- It acts as a bleaching agent for delicate material.
- It is used in the production of epoxides, inorganic chemicals like sodium perborate.
- It is used as an antiseptic for washing wounds.
- It is used in the laboratory for detecting the presence of chromium, titanium, etc.
Uses of H202:
(i) It is used as a mild disinfectant. It is marketed as perhydrol (an Antiseptic).
(ii) It is used in the manufacture of high quality detergents.
(iii) It is used in the synthesis of hydroquinone tartaric acid and certain food products and pharmaceuticals.
(iv) It is used as bleaching agent for textilies, paper pulp etc.
(v) It is used for pollution control treatment of domestic and industrial effluents.
(vi) 93% H202 is used as an oxidant for rocket fuel.
8. Heavy water
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
HEAVY WATER (D20)
It is used in the preparation of other deuterium compounds.
![]()
Uses of D2O:
(i) It is used as moderator in nuclear reactors.
(ii) It is used in the exchange reaction study of reaction mechanisms.
9. Dihydrogen as a fuel
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
HYDROGEN AS A FUEL
Hydrogen Economy: The basic principle of hydrogen economy is the transportation and storage of energy in the form of liquid or gaseous dihydrogen. Advantage is that energy is transmitted in the form of dihydrogen and not as electric power.
Advantage as a fuel:
- It is used as fuel cells for the generation of electric power.
- One major advantage of combustion of hydrogen is that it produces very little pollution and there is not any emission of unbumt carbon particles in the form of smoke.
- It is evident from the study that dihydrogen in the gaseous state as well as in liquefied form releases more energy on combustion as compared to the other fuel commonly used.
- 5% of dihydrogen is mixed in CNG for use in four wheeler vehicles.
1. Group I elements : Alkali metals
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
CHAPTER -10 THE S-BLOCK ELEMENTS
• General Electronic Configuration of s-Block Elements
For alkali metals [noble gas] ns1
For alkaline earth metals [noble gas] ns2
ALKALI METALS
Electronic Configuration, ns1, where n represents the valence shell.
These elements are called alkali metals because they readily dissolve in water to form soluble hydroxides, which are strongly alkaline in nature.
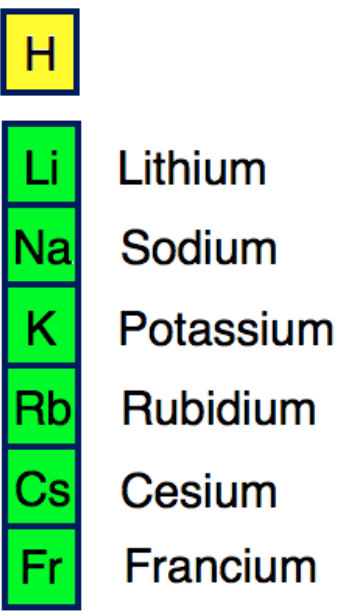
• Atomic and Ionic Radii
Atomic and ionic radii of alkali metals increase on moving down the group i.e., they increase in size going from Li to Cs. Alkali metals form monovalent cations by losing one valence electron. Thus cationic radius is less as compared to the parent atom.
• Ionization Enthalpy
The ionization enthalpies of the alkali metals are generally low and decrease down the group from Li to Cs.
Reason: Since alkali metals possess large atomic sizes as a result of which the valence s-electron (ns1) can be easly removed. These values decrease down the group because of decrease in the magnitude of the force of attraction with the nucleus on account of increased atomic radii and screening effect.
• Hydration Enthalpy
Smaller the size of the ion, more is its tendency to get hydrated hence more is the hydration enthalpy.
Hydration enthalpies of alkali metal ions decrease with increase in ionic sizes.
Li+ > Na+ > K+ > Rb+ > Cs+
• Physical Properties
(i) All the alkali metals are silvery white, soft and light metals.
(ii) They have generally low density which increases down the group.
(iii) They impart colour to an oxidising flame. This is because the heat from the flame excites the outermost orbital electron to a higher energy level. When the excited electron comes back to the ground state, there is emission of radiation in the visible region.
• Chemical Properties of Alkali Metals
(i) Reaction with air:
When exposed to air surface of the alkali metals get tarnished due the formation of oxides and hydroxides.
Alkali metals combine with oxygen upon heating to form different oxides depending upon their nature.
e.g. 4Li + O2 → 2Li2O
Lithium Oxide
2Na + O2 → Na2O2
Sodium Peroxide
K + O2 → KO2
Potassium Superoxide
(ii) Reaction with water:
Alkali metals react with water to form hydroxide and dihydrogen
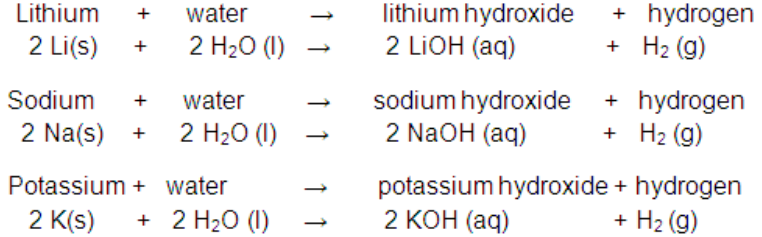
(iii) Reaction with hydrogen:
The alkali metals combine with hydrogen at about 673 K (lithium at 1073 K) to form hydrides.
2M + H2 ————-> 2M+
The ionic character of hydrides increases from Li to Cs.
(iv) Reaction with halogens:
Alkali metals combine with halogens directly to form metal halides.
2M + X2————–> 2MX
They have high melting and boiling points.
Order of reactivity of M:
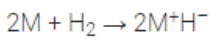
(v) Reducing nature:
The alkali metals are strong reducing agents. In aqueous solution it has been observed that the reducing character of alkali metals follows the sequence Na < K < Rb < Cs < Li, Li is the strongest while sodium is least powerful reducing agent. This can be explained in terms of electrode potentials (E°). Since the electrode potential of Li is the lowest. Thus Li is the strongest reducing agent.
(vi) Solubility in liquid ammonia:
The alkali metals dissolve in liquid ammonia to give deep blue solution. The solution is conducting in nature.
M+ (x + y) NH3 ———-> [M (NH3) X]+ + [e (NH3) y]–
When light falls on the ammoniated electrons, they absorb energy corresponding to red colour and the light which emits from it has blue colour. In concentrated solution colour changes from blue to bronze. The blue solutions are paramagnetic while the concentrated solutions are diamagnetic.
• Uses of Alkali Metals
Uses of Lithium
(i) Lithium is used as deoxidiser in the purification of copper and nickel.
(ii) Lithium is used to make both primary and secondary batteries.
(iii) Lithium hydride is used as source of hydrogen for meteorological purposes.
(iv) Lithium aluminium hydride (LiAlH4) is a good reducing agent.
(v) Lithium carbonate is used in making glass.
Uses of Sodium
(i) Used as sodium amalgum in laboratory (synthesis of organic compounds).
(ii) Sodium is used in sodium vapour lamp.
(iii) In molten state, it is used in nuclear reactors.
(iv) An alloy of sodium-potassium is used in high temperature thermometres.
Uses of Potassium
(i) Salts of potassium are used in fertilizers.
(ii) Used as reducing agent.
Uses of Cesium
(i) In rocket propellent
(ii) In photographic cells.
2. Characteristics of compounds of alkali metals
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
GENERAL CHARACTERISTICS OF THE COMPOUNDS OF ALKALI METALS
(a) Oxides: Alkali metals when burnt in the air form oxides. The nature of oxides depends upon the nature of the alkali metal.
Under ordinary conditions, lithium forms the monoxide (Li2O), sodium forms the peroxide (Na2O2) and the other alkali metals form mainly superoxides (MO2) along with a small number of peroxides.
The increasing stability of the peroxide or superoxide, as the size of the metal ion increases, is due to the stabilization of large anions by larger cations through lattice energy effects. These oxides are easily hydrolysed by water to form the hydroxides according to the following reactions:
M2O + H2O → 2M+ + 2OH–
M2O2 + 2H2O → 2M+ + 2OH– + O2
2MO2 + 2H2O → 2M+ + 2OH– + H2O2 + O2
The oxides and the peroxides are colourless, but the superoxides are yellow or orange coloured. The superoxides are also paramagnetic. Sodium peroxide is widely used as an oxidizing agent in inorganic chemistry.
(b) Hydroxides: Alkali metal hydroxides, MOH are prepared, by dissolving the corresponding oxide in water. Their solubility in the water further increases as we move down the group due to a decrease in lattice energy.
Properties:
1. These are white crystalline solid, highly soluble in water and alcohols. Their solubility in the water further increases as we move down the group due to a decrease in lattice energy.
2. Since alkali metals are highly electropositive, their hydroxides form the strongest bases known. They dissolve in water with the evolution of much heat to give a strongly alkaline solution.
3. They melt without decomposition and are good conductors of electricity in the fused state.
4. These are stable to heat and do not lose water even at red heat. The thermal stability increases on moving from Li to Cs. However, they sublime at about 400°C and the vapours mainly consists of dimers. (MOH)2.
(c) Halides: Alkali metal halides arc prepared by the direct combination of the element, M and halogens. They are normally represented by the formula MX and Cs and Rb, being of large size, also form Polyhalides, i.e. Csl3
Properties:
1. All alkali halides except lithium fluoride are freely soluble in water (LiF is soluble in non-polar solvents).
2. They have high melting and boiling points.
3. Solubility of halides of alkaline metals: The solubility of alkali metal halides show a gradation. For example

4. They are good conductors of electricity infused state.
5. They have an ionic crystal structure. However, lithium halides have a partly covalent character due to polarising power of Li+ ions.
(d) Carbonates and bicarbonates: All alkali metals from carbonates of the type M2CO3. Due to the high electropositive nature of the alkali metals, their carbonates (and also the bicarbonates) are highly stable to heat (however, lithium carbonate decomposes easily by heat. Further, as the electropositive character increases in moving down the group, the stability of carbonates (and bicarbonates) increases in the same order.
Both carbonates and bicarbonates are quite soluble in water and their solubility increases as we move down the group from Li to Cs. Since carbonates are salts of a weak acid (carbonic acid H2CO3), they are hydrolysed in water to give a basic solution.
2M+ + CO3– + H – OH = 2M+ + HCO32- + OH–
Since the alkali metals are highly electropositive, these are the only elements that form stable solid carbonates. However, lithium due to its less electropositive nature does not form solid bicarbonate.
(e) Hydrides: Alkaline metals form hydrides of the type M+N–. The presence of hydrogen as an anion in alkali metal hydrides is evidenced by the fact that on electrolysis hydrogen is liberated at the anode. The hydrides are not very stable. They react with water liberating hydrogen
LiH + H2O → LiOH + H2
These hydrides are, therefore, used as reducing agents. Lithium aluminium hydride, LiAlH4 is even a stronger reducing agent and is used in organic chemistry.
3. Anomalous properties of lithium
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ANOMALOUS BEHAVIOUR OF LITHIUM
Lithium shows anomalous behaviour due to the following reasons:
1. It has the smallest size in its group.
2. It has very high ionization enthalpy and highest electronegativity in the group.
3. Absence of d-orbitals inits valence shell.
As a result, it differs from the other alkali metals in the following properties :
- Lithium is harder than other alkali metals, due to strong metallic bond.
- Lithium combines with O2 to form lithium monoxide, Li2O whereas other alkali metals form peroxides (M2O2) and superoxides (MO2).
- Lithium, unlike the other alkali metals, reacts with nitrogen to form the nitride.
6Li + N2 → 2Li3N
Lithium nitride
- Li2CO3 ,LiF and lithium phosphate are insoluble in water while the corresponding salts of other alkali metals are soluble in water.
- Li2CO3 decomposes on heating to evolve CO2 whereas other alkali metal carbonates do not.
- Lithium nitrate on heating evolves O2 and NO2 and forms Li2O while other alkali metal nitrates on heating form their respective nitrites.
Diagonal Relationship
Lithium shows diagonal resemblance with magnesium [the element of group 2] and this resemblance is due to similar polarising power, i.e.,
[ionic charge / (ionic radius)2] of both these elements.
Lithium resembles magnesium in the following respects :
- The atomic radius of lithium is 1.31 Å while that of magnesium is 1.34 Å.
- The ionic radius of Li+i on is 0.60 Å, which is very close to that of Mg2+ ion (0.65 Å).
- Lithium (1.0) and magnesium (1.2) have almost similar electronegativities.
- Both Li and Mg are hard metals.
- LiF is partially soluble in water like MgF2.
- Both combine with O2 to form monoxides, e.g., Li2O and MgO.
- Both LiOH and Mg(OH)2 are weak bases.
- Both LiCI and MgCl2 are predominantly covalent.
- Both Li and Mg combine with N2 to form their respective nitrides, Li3N and Mg3N2.
Both lithium and magnesium nitrates on heating evolve NO2 and O2 leaving behind their oxides.
4. Some important compounds of sodium
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
SOME IMPORTANT COMPOUNDS OF SODIUM
1. Sodium Chloride, Common Salt or Table Salt [NaCI]
Sea water contains 2.7 to 2.9%by mass of the salt. Sodium chloride is obtained by evaporation of sea water but due to the presence of impurities like CaCl2 and MgCl2 it has deliquescens nature. It is purified by passing HCI gas through the impure saturated solution of
NaCl and due to common ion effect, pure NaCl gets precipitated. 28% NaCl solution is called brine.
2. Sodium Hydroxide or Caustic Soda [NaOH]
Methods of preparation
(i) A 10% solution of Na2CO3 is treated with milk of lime (Causticizing process).
Na2CO3 + Ca(OH)2 → CaCO3 ↓ + 2NaOH
(ii) Electrolytic process involves Nelson cell and Castner-Kellner cell.
A brine solution is electrolysed using a mercury cathode and a carbon anode. Sodium metal discharged at the cathode combines with Hg to form Na-amalgam. Chlorine gas is evolved at the anode.
The amalgam is treated with water to give sodium hydroxide and hydrogen gas.
2Na-Hg + 2H2O → 2NaOH + 2Hg + H2
Physical properties
Sodium hydroxide is a white translucent solid. It is readily soluble in water. Crystals of NaOH are deliquescent.
Chemical properties
1. It is a hygroscopic, deliquescent white solid, absorbs CO2 and moisture from the atmosphere
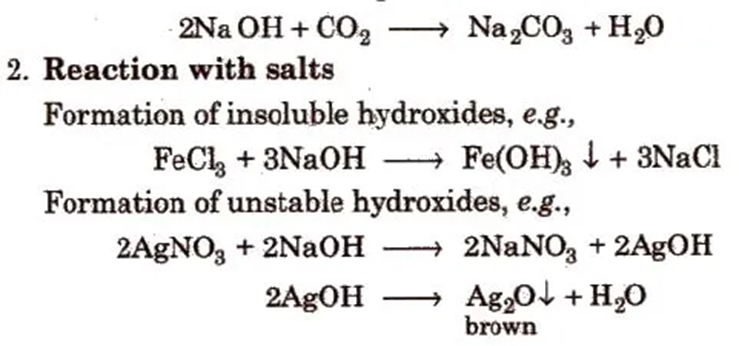
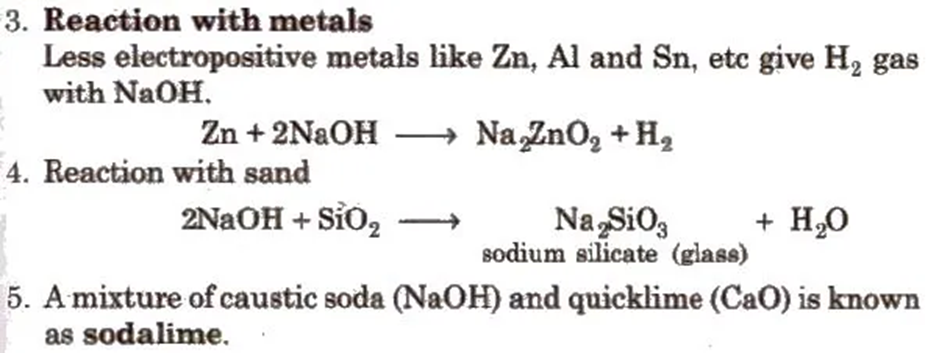
3. Sodium Carbonate or Washing Soda (Na2CO3 . 10H2O)
Solvay process
CO2 gas is passed through a brine solution saturated with NH3
Sodium bicarbonate is filtered and dried. It is ignited to give sodium carbonate.
![]()
Properties
1. Sodium carbonate crystallises from water as decahydrate which effloresces on exposure to dry air forming monohydrate which on heating change to anhydrous salt (soda-ash).
Uses
1. It is used in water softening, laundering and cleaning.
2. It is used in paper, paints and textile industries
4. Sodium Bicarbonate or Baking Soda (NaHCO3) Preparation
It is obtained as an intermediate product in Solvay process.
Properties
Uses
1. It is used as a constituent of baking powder which is a mixture of sodium bicarbonate, starch and potassium bitartrate or cream of tartar and in medicine to remove acidity of the stomach (as antacid).
2. NaHCO3 is a mild antiseptic for skin infections.
3 It is used in fire extinguisher.
5. Microcosmic salts [Na(NH4)HPO4 . 4H2O]
Preparation
It is prepared by dissolving Na2HPO4 and NH4Cl in the molecular proportions in hot water followed by crystallisation.
Properties
On heating it forms a transparent glassy bead of metaphosphate, which gives coloured beads of orthophosphates when heated with coloured salts like that of transition metal ions(Cu2+, Fe2+, Mn2+, Ni2+, CO2+).
This test is called microcosmic bead test.
Na(NH4) HPO4 → NH3 + H2O + NaPO3
sodium metaphosphate
CUSO4 → CuO + SO3
CuO + NaPO3 → CuNaPO4 (blue bead)
It is especially used to detect silica which being insoluble in NaPO3 gives a cloudy bead.
5. Biological importance of sodium and potassium
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
BIOLOGICAL IMPORTANCE OF SODIUM AND POTASSIUM
1. Sodium ions help to regulate flow of water across the cell membranes, transportation of sugars and amino acids inside the cells.
2. Potassium ions activate many enzymes, takes part in oxidation of glucose.
3. Potassium ions together with sodium ions are responsible for transmission of nerve signals and maintenance of ionic gradient across the cell.
6. Group II elements : Alkaline earth metals
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ALKALINE EARTH METALS
Alkaline Earth Metals: They were named alkaline earth metals since they were alkaline in nature like alkali metals oxides and they were found in the earth’s crust.
Example, Be (Beryllium), Ca, Mg, Sr etc.
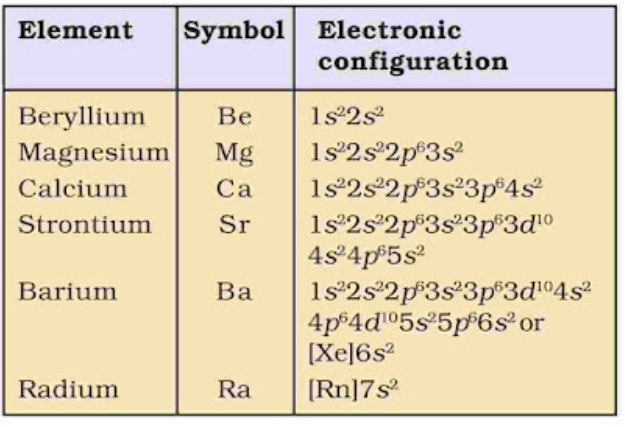
• Electronic Configuration
Their general electronic configuration is represented as [noble gas] ns2.
• Atomic and Ionic Radii
Atomic and ionic radii of alkaline earth metals one comparatively smaller than alkali metals. Within the group atomic and ionic radii increases with the increase in atomic number. Reason: Because these elements have only two valence electrons and the magnitude of the force of attraction with the nucleus is quite small.
• Ionization Enthalpies
These metals also have low ionization enthalpies due to fairly large size of atoms. As the atomic sizes increase down the group ionization enthalpies are expected to decrease in the same manner.
Due to their small size in comparison to alkali metals first ionization enthalpies of alkaline earth metals is higher than that of alkali metals.
• Hydration Enthalpies
The hydration enthalpies of alkaline earth metal ions are larger than those of the alkali metals. Thus alkaline earth metals have more tendency to become hydrate. The hydration enthalpies decreases down the group since the cationic size increases.
Be2+ > Mg2+ > Ca2+ > Sr2+ > Ba2+
Metallic character: They have strong metallic bonds as compared to the alkali metals in the same period. This is due to the smaller kernel size of alkaline earth metal and two valence electrons present in the outermost shell.
• Physical Properties
(i) They are harder than alkali metals.
(ii) M.P and B.P are higher than the corresponding alkali metals due to their small size.
(iii) The electropositive character increases down the group.
(iv) Except Be and Mg, all these metals impart characteristic colour to the flame.
(v) The alkaline earth metals possess high thermal and electrical conductivity.
• Chemical Properties
1. Reaction with oxygen. Beryllium and magnesium are kinetically inert to oxygen because of the formation of a thin film of oxide on their surface.
Reactivity towards oxygen increases as going down the group.
2. Reaction with water. Since these metals are less electropositive than alkali metals, they are less reactive towards water.
Magnesium reacts with boiling water or steam. Rest of the members reacts even with cold water.
Mg + 2H20 ——-> Mg(OH)2 + H2
Ca + 2H20 ————> Ca(OH)2 + H2
3. Reaction with halogens. They combine with the halogens at appropriate temperature to form corresponding halides MX2.
M + X2 ——–> MX2 (X = F, Cl, Br, I)
Thermal decomposition of (NH4)2 BeF4is used for the preparation of BeF2.
4. Reaction with hydrogen. These metals except Be combine with hydrogen directly upon heating to form metal hydrides.
BeH2, however, can be prepared by the reaction of BeCl2 with LiAlH4.
2BeCl2 + LiAlH4 → 2BeH2 + LiCl + AlCl3
7. General characteristics of the compounds of the alkaline earth metals
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
GENERAL CHARACTERISTICS OF COMPOUNDS OF ALKALINE EARTH METALS
Oxides and Hydroxides
(i) The alkaline earth metals bum in oxygen to form MO (monoxide).
(ii) These oxides are very stable to heat.
(iii) BeO is amphoteric in nature while oxides of other elements are ionic.
(iv) Exept BeO, they are basic in nature and react with water to form sparingly soluble hydroxides.
MO + H2O ———> M(OH)2
(v) Hydroxides of alkaline earth metals are less stable and less basic than alkali metal hydroxides.
(vi) Beryllium hydroxide is amphoteric in nature.
Halides
The alkaline earth metals combine directly with halogens at appropriate temperatures forming halides, MX2.
They can also be prepared by the action of halogen acids (HX) on metals, metal oxides, metal hydroxides.
M + 2HX ——-> MX2 + H2
MO + 2HX ——> MX2 + H20
M (OH)2 + 2HX —–> MX2 + 2H20
(i) Except beryllium halides, all other halides of alkaline earth metals are ionic in nature.
(ii) Except BeCl2 and MgCl2 other chloride of alkaline earth metals impart characteristic colours to flame.
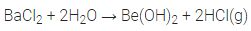
(iii) The tendency to form halide hydrates decreases down the group.
For example, (MgCl2– 8 H20, CaCl2– 6 H20, SrCl2– 6 H20, BaCl2– 2 H2O)
(iv) BeCl2 has a chain structure in the solid phase as shown below.
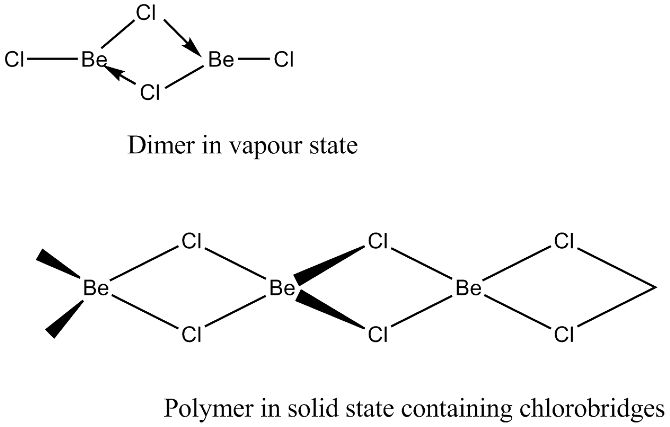
In vapour phase the compound exist as a dimer which decomposes at about 1000K to give monomer in which Be atom is in sp hybridisation state.
Sulphates
(i) The sulphates of alkaline earth metals are white solids and quite stable to heat.
(ii) BeS04 and MgS04 are readily soluble in water. Solubility decreases from BeS04 to BaS04.
Reason. Due to greater hydration enthalpies of Be2+ ions and Mg2+ ions they overcome the lattice enthalpy factor. Their sulphates are soluble in water.
8. Anomalous properties of beryllium
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ANOMALOUS BEHAVIOUR OF BERYLLIUM
The anomalous behaviour of beryllium is mainly died to its very small size and partly due to its high electronegativity. These two factors increase the polarising power [Ionic charge/ (ionic radii)2] of Be2+ ions to such extent that it becomes significantly equal to the polarising power of Al3+ ions.
Hence the two elements resemble (diagonal relationship) very much.
1. Both of them have the same value of electronegativity (1.5).
2. The standard oxidation potential of Be and Al are of the same order (Be = 1.69 V, Al = 1.7 V)
3. In nature both occur together in beryl, 3BeO, Al2O3, 6SiO2.
4. Due to its small size, beryllium has a high charge density and therefore, exhibits a strong tendency to form covalent compounds. Aluminium too has a strong tendency to form covalent compounds. Thus salts of both beryllium and aluminium have low m.p. are soluble in organic solvents and get hydrolysed by water.
Beryllium does show some tendency to form covalent compounds but other alkaline earth metals do not form covalent compounds.
5. Unlike other alkaline earth metals but like aluminium, beryllium is not easily affected by dry air.
6. Both (Be and Al) do not decompose water even on boiling; because of their weak electropositive character. Other alkaline earth » metals decompose even cold water evolving hydrogen.
7. Beryllium, like aluminium, reacts very slowly with dilute – mineral acids liberating hydrogen.
Be + 2HCl → BeCl2 + H2
2Al + 6HCl → 2AlCl3 + 3H2
Other alkaline earth metals react very readily with dilute acids.
8. The chlorides of both beryllium and aluminium have bridged chloride structures in the vapour phase.
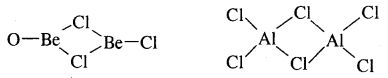
9. Salts of these, metals form hydrated ions e.g., [Be(OH2)4]3+ and [Al(OH2)6]3+ in aqueous solutions.
10. Beryllium and aluminium both react with caustic alkalies to form beryllate and aluminate respectively. Other alkaline earth metals do not react with caustic alkalies.
9. Some important compounds of calcium
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
SOME IMPORTANT COMPOUNDS OF CALCIUM
(i) Calcium Oxide or Quick Lime, CaO

Uses:
(i) In the manufacture of cement, sodium carbonate, calcium carbide etc.
(ii) Used in the purification of sugar.
(iii) In the manufacture of dye stuffs.
(ii) Calcium Hydroxide (Slaked lime), Ca(OH)2
Carbonates
Carbonates of alkaline earth metals are thermally unstable and decompose on heating.
Calcium hydroxide is prepared by adding water to quick lime, CaO. It is a white amorphous powder. It is sparingly soluble in water. The aqueous solution is known as lime water and a
suspension of slaked lime in water is known as milk of lime. When carbon dioxide is passed through lime water it turns milky due to the formation of calcium carbonate.
Uses:
(i) It is used in the manufacturing of building material.
(ii) Used in white-wash as a disinfectant.
(iii) Used to detect C02 gas in the laboratory.
(iii) Calcium Carbonate or Limestone (CaC03)
Preparation: Calcium carbonate occurs in nature in different forms like limestone, marble, chalk etc. It can be prepared by passing C02 through slaked lime in limited amount.
Ca(OH)2 + C02 ——> CaC03 + H20
It can also prepared by the reaction of a solution of sodium carbonate with calcium chloride.
CaCl2 + Na2C03 ——> CaC03 + 2NaCl
Uses:
(i) In the manufacturing of Quick Lime.
(ii) With MgC03 used as flux in the extraction of metals.
(iii) Used as an antacid.
(iv) In the manufacture of high quality paper.
(iv) Calcium Sulphate (Plaster of Paris) CaS04-1/2H20
Preparation: It is obtained when gypsum CaS04– 2 H20 is heated to 393 K
2(CaS04-2H20) ———-> 2(CaS04) . H20 + 3H20
Above 393 K anhydrous CaS04 is formed, which is called ‘dead burnt plaster’.
Properties:
(i) It is a white atmosphous powder.
(ii) When it is mixed in adequate quantity of water it forms a plastic hard mass within 15 minutes.
Uses:
(i) Commonly used in making pottery, ceramics etc.
(ii) Used in the surgical bandages for setting the fractured bone or sprain.
(iii) For making statues, ornamental work, decorative material etc.
(v) Cement
Preparation: Prepared by combining a material rich in CaO with other material such as clay, which contains Si02 along with the oxides of aluminium, iron and magnesium.
Important Ingredients of portland cement:
(Ca2Si04) dicalcium silicate 26%
(Ca2SiO4) Tricalcium silicate 51%
(Ca3Al206) Tricalcium Aluminate 11%
Uses:
In plastering and in construction purposes.
• s-block elements constitute Group I and II elements.
• General electronic configuration of
Group I = [Noble gas] ns1
Group II = [Noble gas] ns2
• Diagonal Relationship
The first three elements of second period (Li, Be, B) show diagonal similarity with the elements (Mg, Al, Si) of third period. Such similarities are termed as diagonal relationship.
• The alkali metals are silvery-white soft metals. They are highly reactive. Their aqueous solutions are strongly alkaline in nature. Their atomic and ionic sizes increase on moving down the group and ionization enthalpies decrease systematically down the group.
• Alkaline earth metals. They are much similar to alkali metals but due to small size some differences are there. Their oxides and hydroxides are less basic than the alkali metals.
• Sodium hydroxide (NaOH) is prepared by the electrolysis of aq NaCl in Castner- Kellner cell.
Slaked lime Ca(OH)2 is formed by the action of quick lime on water.
• Gypsum is CaS04. 2 H20. On heating upto 390 K CaS04/2H20 (plaster of paris) is formed.
10. Biological importance of magnesium and calcium
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
BIOLOGICAL IMPORTANCE OF MAGNESIUM & CALCIUM
1. An adult body contains about 25g of Magnesium and 1200 g of calcium as compared with only 5g of iron and 0.06g of copper. The daily requirement in the human body has been estimated to be 200-300 mg.
2. All enzymes that utilize ATP in phosphate transfer require magnesium as the cofactor.
3. The main pigment for the absorption of light in plants for photosynthesis is green coloured chlorophyll which contains magnesium.
4. About 99% of body calcium is present in bones and teeth. It also plays important roles in neuromuscular function, interneuronal transmission, cell membrane integrity and blood coagulation. The calcium concentration in plasma is regulated at about 100 mg L-1. It is maintained by two hormones.
5. Calcitonin and parathyroid hormone. Bone is not an inert and unchanging substance but is continuously being solubilized and redeposited to the extent of 400 mg per day in man. All the calcium passes through the plasma.
2. Important trends and anomalous properties of boron
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
IMPORTANT TRENDS AND ANOMALOUS PROPERTIES OF BORON
Trichloride, Tribromides and Triiodides of group 13 elements are covalent in nature and can be hydrolysed in water.
Monomeric trihalides of these elements are strong Lewis acids.
BF3 + :NH3 → NH3-BF3
Metal halides of group 13 elements are generally dimerized through halogen bonding.
Anomalous properties of Boron are listed below –
Boron shows quite higher melting and boiling points than other elements of group 13.
Boron forms only covalent compounds while other elements of the group 13 form both ionic and covalent compounds.
Boron is a metalloid while other elements of the group 13 are metals.
Oxides and hydroxides of boron are acidic in nature while oxides and hydroxides of other elements of the group are amphoteric and basic in nature.
3. Some important compounds of boron
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
SOME COMPOUNDS OF BORON
Structure of boric acid
It has a layer structure in which planar BO3 units are joined by hydrogen bonds as shown in Fig.
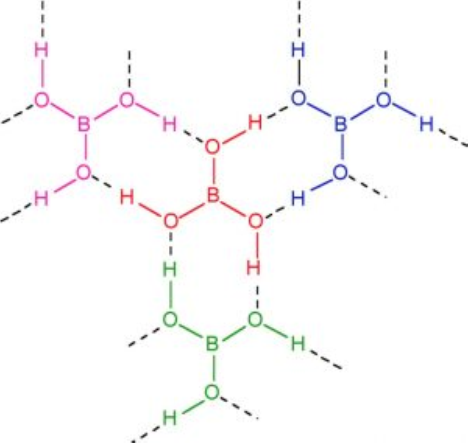
Physical properties of boric acid:
(i) It is a white crystalline solid.
(ii) It is soft soapy in touch.
(iii) It is sparingly soluble in cold water but fairly soluble in hot water.
Uses:
(i) In the manufacture of heat resistant borosilicate glazes.
(ii) As a preservative for milk and food stuffs.
(iii) In the manufacture of enamels and glazes in pottery.
(iii) Diborane, (B2H6): The series of compounds of boron with hydrogen is known as boranes.
Diborane is prepared by the reduction of boron trifluoride with LiAlH4 in diethyl ether.
4BF3 + 3LiAlH4 ——> 2B2H6+ 3LiF + 3AlF3
Laboratory method of preparation. In laboratory diborane is prepared by the oxidation of sodium borohydride with iodine.
2NaBH4 + I2 ——> B2H6 + 2NaI +H2
Industrial method of preparation. On industrial scale, diborane is prepared by reduction of BF3 with sodium hydride.
![]()
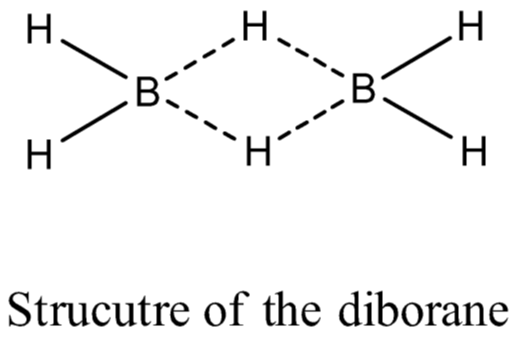
Physical Properties:
(i) Diborane is a colourless, highly toxic gas with a b.p. of 180 K.
(ii) Diborane catches fire spontaneously upon exposure to air.
(iii) Higher boranes are spontaneously flammable in air.
Chemical properties:
(i) Boranes are readily hydrolysed by water to form boric acid
B2H6(g) + 6H20(Z) ——> 2B(OH)3(aq) + 6H2(g)
(ii) It burns in oxygen evolving an enormous amount of heat
B2H6 + 302 —–> B203 + 3H20
(iii) Reaction with Lewis base:
Diborane on treatment with lewis bases undergo cleavage reactions to form borane which then reacts with Lewis bases to form adducts.
B2H6 + 2NMe3 ——> 2BH3. NMe3
B2H6 + 2CO ———> 2BH3 .CO
4. Uses of boron and aluminium and their compounds
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
USES OF BORON AND ALUMINIUM AND THEIR COMPOUNDS
Uses of Boron
1) Boron fibres have enormous tensile strength and hence are used to make bullet proof vests and light composite material for aircraft.
2) Natural boron is 20% boron-10 and 80% boron-11.Boron-10 has a high cross section for absorption of low energy neutrons.
3) Borax and boric acid are used in the manufacture of heat resistant glass, glass wool and fibre glass. Borax is also used as a flux in soldering ,as a constituent of medicinal soaps due to its antiseptic properties ,in the manufacture of enamels and glazes for earthenware’s i.e. tiles, pottery etc. An aqueous solution of orthoboric acid is also used as a mild antiseptic.
4) It is used in steel industry for increasing the hardness of Steel. In fact boron has replaced expensive metals like Mo, Cr and W in with manufacture of special hard steels.
5) Boron compounds are being used as rocket fuel because of high energy/ mass ratio.
Uses of aluminium
Aluminium is a bright silvery white metal with high tensile strength.
1) It is used for making transmission cables and for winding the moving coils of dynamos or motors.
2) It is used for making aluminium paint for protection of iron and zinc. Aluminium powder mixed with linseed oil shines like silver and hence is called silver paint.
3) Aluminium is a cheap metal which resist corrosion. Therefore ,it is used for making household utensils ,cans for drinks, tubes for toothpaste, pictures frames, trays etc. It is also used in building for making angles for doors, windows.
4) Aluminium foil is used for wrapping fine articles like photographic films , pharmaceutical products ,cigarettes sweets.
5) Aluminium powder is used as a reducing agent in aluminothermic process for extraction of chromium and manganese from their ores.
6) Aluminium powder is used in flash light bulbs for indoor photography.
7) Large amount of aluminium are converted into alloys. Some important alloys of aluminium are Aluminium bronze (used in coins, utensils, jewellery, picture frame), Magnalium (used in light instruments, balance beams, pressure cookers) , duralumin (used in automobile parts, pressure cooker)
8) Al(OH)3 is widely used as an antacid for treatment of digestion.
9) Anhydrous AlCl3 is used as a catalyst in Friedel – Crafts reaction and in cracking of petroleum. Hydrated AlCl3 is used as a mordant in dyeing.
10) Potash alum is used for purification of water, as styptic for stopping bleeding , in form type fire extinguisher, as mordant for dyeing and for tanning of leather and sizing of paper.
5. Group 14 elements : The carbon family
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
GROUP 14 ELEMENTS: THE CARBON FAMILY
Group 14 includes carbon (C), silicon (Si), Germanium (Ge), tin (Sn) and lead (Pb).
General electronic configuration of carbon family is ns2np1.
Carbon: Carbon is the seventeenth most abundant element by weight in the earth’s crust.
(i) It is available as coal, graphite and diamond. In combined state it is present in metal carbonates, hydrocarbons and carbon dioxide gas (0.03%) in air.
(ii) Naturally occurring carbon contains two stable isotopes 12C and 13C and third isotope 14C. 14C is a radioactive isotope with half life 5770 years and is used for radiocarbon dating.
Covalent radius: Covalent radius expected to increase from C to Si. From Si to Pb small increase is found.
Reason: Due to the addition of a new energy shell in each succeeding element. The increase in covalent radii from Si to Pb is small due to ineffective shielding of the valence electrons by the intervening d- and f orbitals.
Ionization Enthalpy: The first ionization enthalpies of group 14 elements are higher than those of the corresponding group 13 elements.
Reason: Because effective nuclear charge increases and size of the atoms becomes smaller. First ionization enthalpy decreases on moving down the group from carbon to tin.
The decrease is very sharp from carbon to silicon while there is slight increase in the first ionization enthalpy of lead as compared to that of tin.
Electronegativity: Group 14 elements are smaller in size as compared to group 13 elements that’s why this group are slightly more electronegative than group 13. From Si to Pb it is almost same. Small increase in ionization enthalpy from Sn to Pb is due to the effect of increased nuclear charge outweighs the shielding effect due to the presence of additional 4f- and 5d-electrons.
Physical properties:
(i) All the elements of group 14 elements are solids. They are less metallic than group 13.
(ii) M.P. and boiling points of group 14 elements are generally high.
Chemical properties:
Carbon and silicon mostly show +4 oxidation state. Germanium forms stable compounds in +4 state and only few compounds in +2 state.
Tin forms compounds in both oxidation states. Lead forms compounds in +2 state are stable and in +4 state are strong oxidising agents.
6. Important trends and anomalous properties of carbon
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
IMPORTANT TRENDS AND ANOMALOUS BEHAVIOUR OF CARBON
Carbon, differs from the rest of the member of its family. The main reason for the anomalous behaviour is:
(i) exceptionally small atomic and ionic size
(ii) higher ionization enthalpy
(iii) absence of d-orbitals in the valence shell.
(iv) Higher electronegativity.
It can be explained as follows:
=> Since carbon has only s and p-orbitals it can accommodate only four pairs of electrons ; other member can expand their covalence due to the presence of d-orbitals.
=> Carbon can form Pπ-Pπ multiple bonds with itself and other atoms having small size and high electronegativity.
For example, C=C, C≡C, C=O, C=S and C≡N
The order of catenation is C >> Si > Ge ≈ Sn
Heavier elements do not form Pπ-Pπ bonds because their atomic orbitals are too
large and diffuse to have effective overlapping.
=> Carbon atoms have the tendency to link with one another through covalent bonds to form chains and rings. This property is called catenation.
Down the group property to show catenation decreases.
C > Si > Ge > Sn > Pb
Lead does not show catenation.
7. Allotropes of carbon
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ALLOTROPES OF CARBON
The property of an element to exist in two or more forms which have different physical properties but identical chemical properties is called allotropy and different forms are called allotropes. Carbon exists in two allotropic forms:
(i) Crystalline
(ii) Amorphous
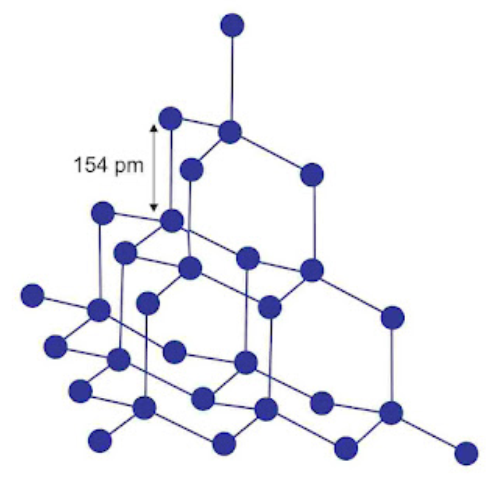
Crystalline form of carbon: Diamond, Graphite, Fullerenes Diamond: In diamond each carbon atom undergoes sp3 hybridisation. Each carbon is tetrahedrally linked to four other carbon atoms. The C—C bond length is 154 pm.
Properties:
(i) It is the hardest substance on earth.
(ii) It is used as an abrasive for sharpening hard tools in making dyes and in manufacture of tungsten filaments.
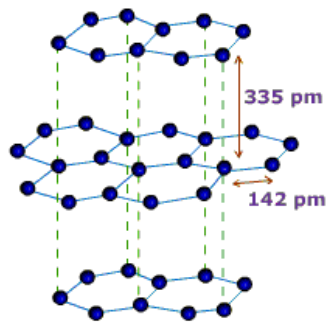
Graphite: In graphite, carbon is sp2-hybridized. Graphite has a two-dimensional sheet like structure consisting of a number of hexagonal rings fused together. Layers are held by van der Waals forces and distance between two layers is 340 pm.
Properties:
(i) Graphite conducts electricity along the sheet.
(ii) It is very soft and slippery.
(iii) Used as a dry lubricant in machines running at high temperature, where oil cannot be used as a lubricant.
Fullerenes: Fullerenes was discovered collectively by three scientists namely E. Smalley, R.F. Curl and H.W. Kroto.
Preparation:
Fullerenes is prepared by heating of graphite in an electric arc in the presence of inert gas such as helium or argon.
The sooty material formed by the condensation of vapourised Cn small molecules consists of mainly with smaller quantity of C70 and traces of other fullerenes consisting of even number of carbon atoms up to 350or above.
Fullerenes are cage like molecules. C60 molecule has a shape like soccer ball and called Buckminsterfullerene’s. It is the most stable.
It contains 20 six-membered rings and 12 five-membered rings.
Six-membered rings are fused to both the other six-membered rings and five-membered rings but the five-membered rings are connected only to six-membered rings.
All the carbon atoms are equal and they undergo sp2-Kybridization.
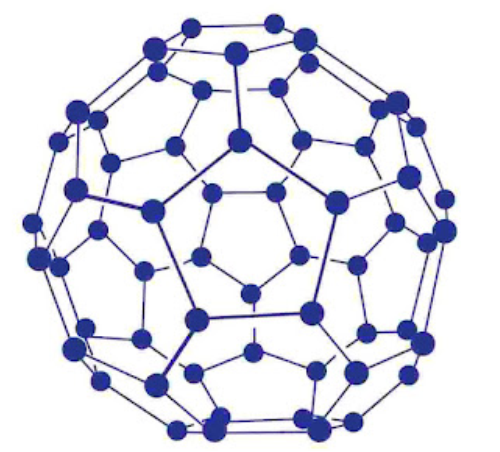
Properties:
(i) Fullerenes being covalent are soluble in organic solvents.
(ii) It also forms platinum complexes.
Amorphous allotropic forms of carbon coke: It is a greyish black hard solid and is obtained by destructive distillation.
Wood charcoal: It is obtained by strong heating of wood in a limited supply of air.
Animal charcoal: It is obtained by the destructive distillation of bones.
Uses of carbon:
(i) Graphite fibre are used for making superior sports goods such as tennis and badminton rackets, fishing rods.
(ii) Being good conductor graphite is used for making electrodes for batteries and industrial electrolysis.
(iii) Being highly porous, activated charcoal is used for absorbing poisonous gases in gas masks. It is used to decolourize sugar.
(iv) Carbon black is used as black pigment in black ink and as filler in automobile tyres.
(v) Coke is extensively used as reducing agent in metallurgy.
(vi) Diamond is a precious stone.
8. Some important compounds of carbon and silicon
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
SOME IMPORTANT COMPOUNDS OF CARBON AND SILICON
Carbon Monoxide
Preparation: It is prepared by direct oxidation of C in limited supply of oxygen.
2C + O2 → 2CO
Properties:
(i) Carbon monoxide is a colourless and odourless gas.
(ii) It is almost insoluble in water.
(iii) It is powerful reducing agent and reduces almost all metal oxides except alkali and alkaline earth metal oxides.
(iv) In CO molecule there are one σ (sigma) and two π bonds between carbon and oxygen.
: C = O :
(v) It is highly porous in nature. It forms a complex with haemoglobin which is about 300 times more stable than the oxygen-haemoglobin complex. This prevents haemoglobin in the red blood corpuscles from carrying oxygen round the body, thereby causing suffocation ultimately leading to death.
Carbon Dioxide
Preparation: It is prepared by complete combustion of carbon and carbon containing fuels in
- CaCO3 + 2HCl → CaCl2 + H2O + CO2
- CaCO3 → CaO + CO2
Structure:
Carbon dioxide, or CO2, has three resonance structures, out of which one is a major contributor.
The CO2 molecule has a total of 16 valence electrons - 4 from carbon and 6 from each oxygen atom.
Here are the three resonance structures for CO2, all accounting for the 16 valence electrons
The atoms in all three resonance structures have full octets; however, structure 1 will be more stable, and thus contribute more, because it has no separation of charge.
Structures 2 and 3 show charge separation caused by the presence of formal charges on both oxygen atoms. Moreover, the presence of a positive charge on oxygen further reduces the stability of these two structures.
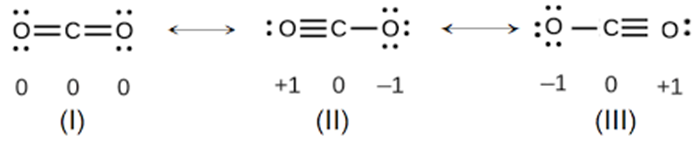
Properties:
(i) It is a colourless and odourless gas.
(ii) It is slightly soluble in water. When C02 dissolves in water only some of the molecules react with water to form carbonic acid.
(iii) It is not poisonous like CO.
But increase in combustion of fossil fuels and decomposition of limestone for cement manufacture increase of C02 in the atmosphere is one of the main reasons of green house effect.
Silicon dioxide (Si02)
Silicon dioxide, commonly known as silica, occurs in various crystallographic forms.
For example, Quartz, Cristobalite and thermite are some of the crystalline forms of silica.
Structure:
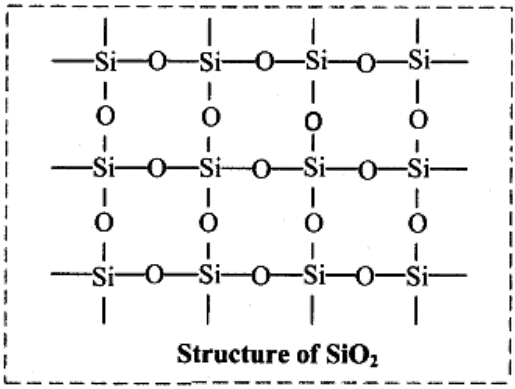
Silicon dioxide is a covalent three dimensional network solid.
Each silicon atom is covalently bonded in a tetrahedral manner to four oxygen atoms.
Each oxygen atom in turn covalently bonded to another silicon atoms as shown below
Properties:
(i) In normal form silica is very less reactive.
(ii) At elevated temperature it does not reacts with halogens, dihydrogen and most of the acids and metals. But it reacts with HF and NaOH.
Si02 + 2NaOH —–> Na2Si03 + H2O
Si02+ 4HF ——–> SiF4+ 2H20
Uses:
(i) Quartz is extensively used as a piezoelectric material.
(ii) Silica gel is used as adsorbent in chromatography.
(iii) An amorphous form of silica, kieselguhr is used in filtration plants.
Zeolites
1. Zeolites are three dimensional crystalline solids containing aluminium, silicon and oxygen in their regular three-dimensional framework.
2. They are hydrated sodium alumino silicates with general formula, Na2O. (Al2O3). x(SiO2)y(H2O) (x = 2 to 10; y = 2 to 6)
3. Zeolites have porous structure in which the monovalent sodium ions and water molecules are loosely held.
4. The Si and Al atoms are tetrahedrally coordinated with each other through shared oxygen atoms.
5. Zeolites structure looks like a honeycomb consisting of a network of interconnected tunnels and cages.
6. Zeolite crystal to act as a molecular sieve. They helps to remove permanent hardness of water.
• P-Block elements: Contains, metals, non-metals and metalloids.
• General configuration: ns2np1-6
– Boron is a typical non-metal and the other members are metals.
– Boron halides are considered to behave like Lewis acids.
– Boric acid is a Lewis acid.
– Borax is a white crystalline solid formula is Na2 [B4O5(OH)4] . 8H20
– Aluminium exhibits +3 oxidation state.
– Allotropy: The important allotropes of carbon are diamond, graphite, and fullerenes.
– The members of carbon family exhibit +4 and +2 oxidation state. The tendency to show +2 oxidation state increases among heavier elements.
– Lead in +2 state is stable whereas in +4 oxidation state it is a strong oxidising agent.
– Carbon monoxide is neutral whereas C02 is acidic in nature.
– Carbon monoxide having lone pair of electrons on C forms metal carbonyls.
– Carbon monoxide forms a haemoglobin complex which is deadly poisonous due to its higher stability.
– Zeolites are complex aluminium silicates.
8. Methods of purification of organic compounds
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
PURIFICATION OF ORGANIC COMPOUNDS
An oganic compound may contain impurities and is essential to purify it. Various methods used for the purification of organic compounds are based on the nature of the compound and the impurity present in it.
The common techniques used for purification are as follows:
- Sublimation
- Crystallisation
- Distillation
- Differential extraction and
- Chromatography
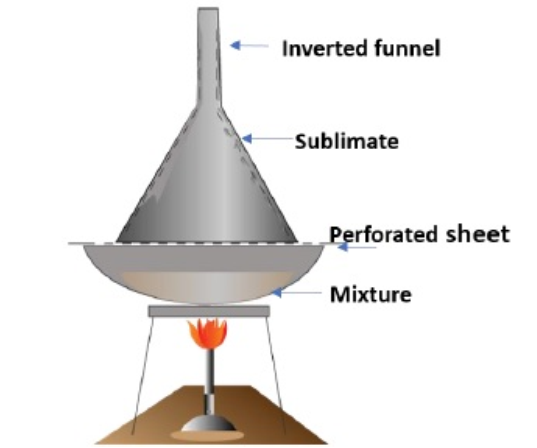
1. Sublimation
- It is the process of conversion of a solid substance directly to vapour by heating.
- It is used to separate sublimable compounds from non-sublimable impurities.
- In this method, the substance is placed in a sublimation apparatus and heated under vacuum.
- Under this reduced pressure, the solid sublimes and condenses as a purified compound on a cooled surface.
- The impurities left behind on the apparatus.
- This method is used for the purification of naphthalene, iodine, camphor etc.
2. Crystallisation
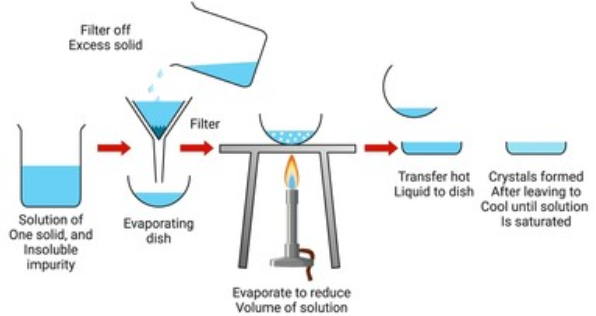
- This is one of the most commonly used techniques for the purification of solid organic compounds.
- It is based on the difference in the solubilities of the compound and the impurities in a suitable solvent.
- The impure compound is dissolved in a solvent in which it is sparingly soluble at room temperature but appreciably soluble at higher temperature.
- The solution is concentrated to get a nearly saturated solution.
- On cooling the solution, pure compound crystallises out and is removed by filtration.
- If the compound is highly soluble in one solvent and very little soluble in another solvent, crystallisation can be satisfactorily carried out in a mixture of these solvents.
3. Distillation
This method is used to separate
(i) volatile liquids from non-volatile impurities and
(ii) the liquids having sufficient difference in their boiling points.
- The principle of this method is that liquids having different boiling points vaporise at different temperatures.
- The vapours are cooled and the liquids so formed are collected separately.
- In this method, the liquid mixture is taken in a round bottom flask and heated carefully.
- On boiling, the vapours of lower boiling liquid are formed first.
- The vapours are condensed by using a condenser and the liquid is collected in a receiver.
- The vapours of higher boiling liquid form later and it can be collected separately.
- Chloroform (b.p 334 K) and aniline (b.p. 457 K) are separated by this technique.
There are different types of distillation methods. They are:
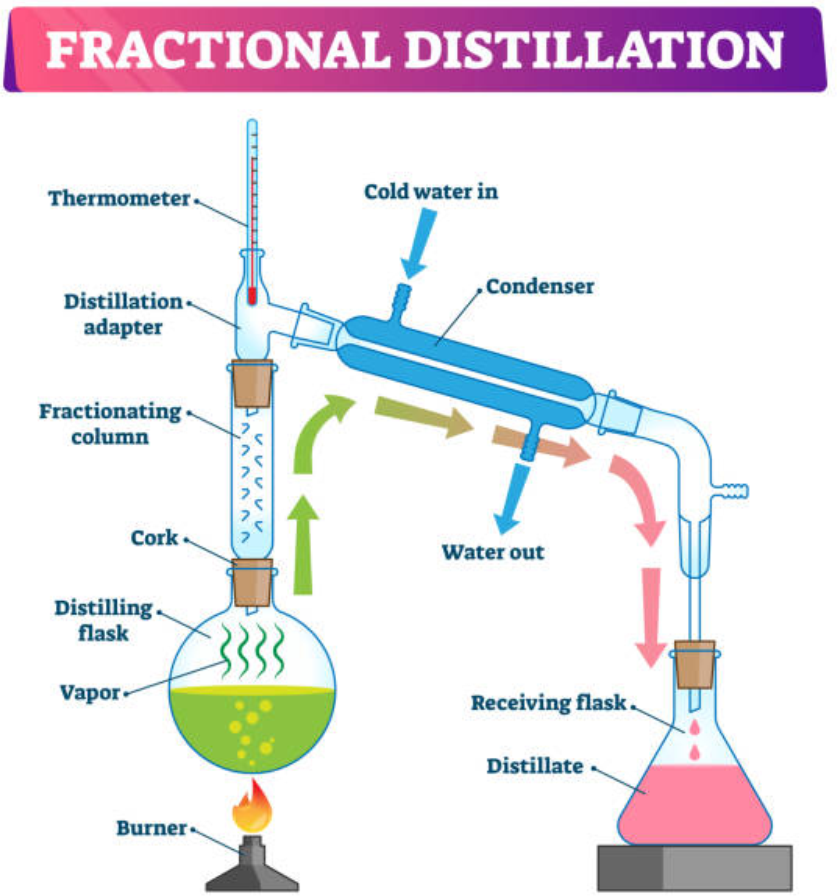
a) Fractional distillation:
- Fractional distillation is used to separate two or more liquids that are miscible.
- It is a special type of distillation designed to separate a mixture of two or more liquids that have different boiling points.
- The process involves heating the mixture and partial condensation of the vapours along a fractionating column.
- The column is set up such that components with lower boiling points pass through the column and are collected earlier than components with higher boiling points.
- Repeated vaporization and condensation result in the separation of the components of the mixture.
- The efficiency of fractional distillation depends on the use of the fractionating column.
- The fractionating column is packed with glass beads.
- It provides a large surface area for vaporization and condensation of the liquid mixture.
- Ethanol and water, crude oil, toluene and cyclohexane etc are separated by this method.
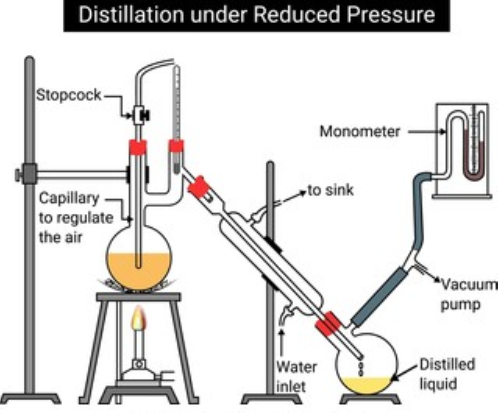
b) Distillation under reduced pressure:
- This method is used to purify liquids having very high boiling points and those, which decompose at or below their boiling points.
- Such liquids are made to boil at a temperature lower than their normal boiling points by reducing the pressure on their surface.
- The pressure is reduced with the help of a water pump or vacuum pump.
- Glycerol can be separated from spent-lye in soap industry by using this technique.
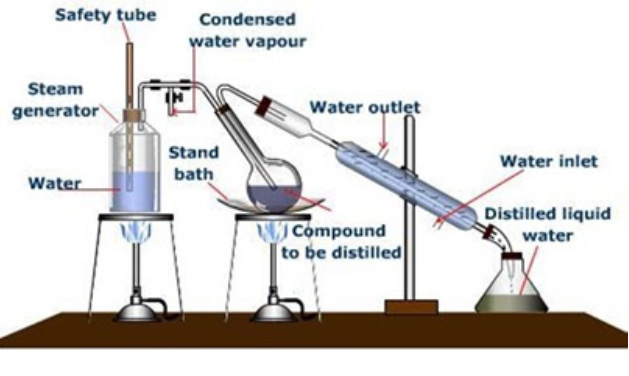
c) Steam Distillation:
- This technique is applied to separate substances which are steam volatile and are immiscible with water.
- In steam distillation, steam from a steam generator is passed through a heated flask containing the liquid to be distilled.
- The mixture of steam and the volatile organic compound is condensed and collected.
- The compound is later separated from water using a separating funnel.
- Aniline – water mixture is separated by this method.
4. Differential Extraction
- When an organic compound is present in an aqueous medium, it is separated by shaking it with an organic solvent in which it is more soluble than in water.
- The organic solvent and the aqueous solution should be immiscible with each other.
- So they form two distinct layers which can be separated by separating funnel.
- The organic solvent is later removed by distillation or by evaporation to get back the compound
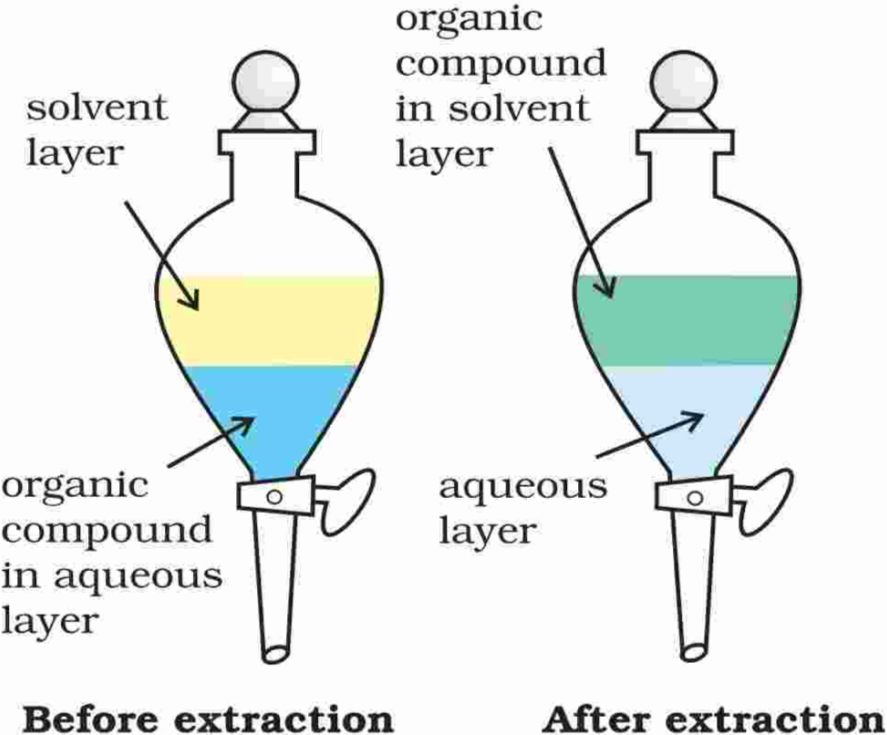
5. Chromatography
- This method is used to separate mixtures into their components, to purify compounds and to test the purity of compounds.
- Here the mixture to be separated is passed through a stationary phase, which may be a solid or a liquid.
- A pure solvent (sometimes a mixture of solvents or a gas) is allowed to move slowly over the stationary phase.
- The moving phase is called the mobile phase.
- The components of the mixture get gradually separated from one another.
Based on the principle involved, there are mainly two types of chromatography:
- Adsorption chromatography, and
- Partition chromatography
a) Adsorption Chromatography
Adsorption chromatography is based on the fact that different compounds are adsorbed on an adsorbent in different degrees. Commonly used adsorbents are silica gel and alumina.
- Here a mobile phase is allowed to move over a stationary phase (adsorbent).
- Based on the adsorbing power, the components of the mixture are adsorbed at different places over the stationary phase.
- Following are two main types of chromatographic techniques based on the principle of differential adsorption.
-
- Column chromatography
- Thin layer chromatography.
1. Column Chromatography:
- It involves the separation of a mixture over a column of adsorbent (stationary phase) packed in a glass tube.
- The column is fitted with a stopcock at its lower end. The mixture to be separated is passed through the column.
- Based on the adsorbing power, the components are adsorbed at different places over the column.
- The most readily adsorbed substances are retained near the top and others come down to various distances in the column.
- Then an appropriate eluant (solvent) is allowed to flow down the column slowly.
- Different solvents are used to separate different components.
- So the components can be collected separately and they can be separated.
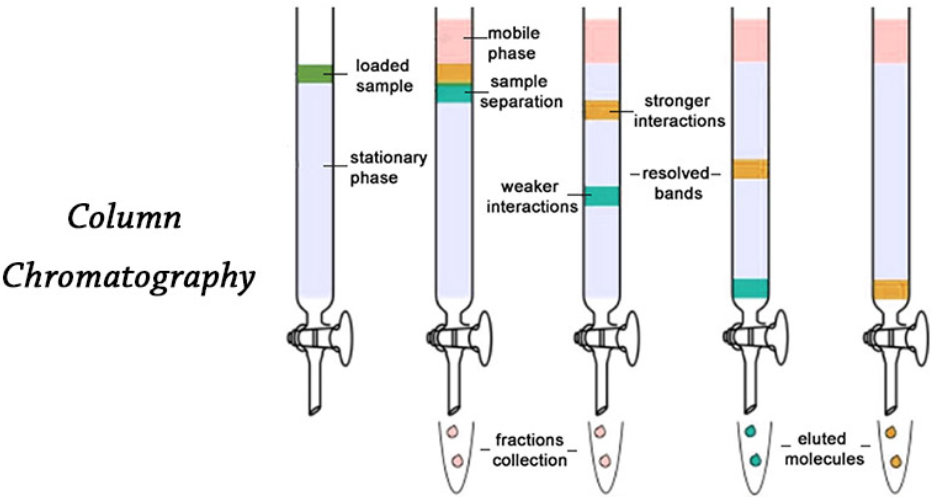
2. Thin Layer Chromatography (TLC):
- It is another type of adsorption chromatography. It involves separation of substances of a mixture over a thin layer of an adsorbent coated on a glass plate.
- A thin layer (about 0.2mm thick) of an adsorbent (silica gel or alumina) is spread over a glass plate of suitable size.
- The plate is known as thin layer chromatography plate or chromaplate.
- The solution of the mixture to be separated is applied as a small spot about 2 cm above one end of the TLC plate.
- The glass plate is then placed in a closed jar containing the eluant.
- As the eluant rises up the plate, the components of the mixture move up along with the eluant to different distances depending on their degree of adsorption and separation takes place.
- The relative adsorption of each component of the mixture is expressed in terms of its retardation factor (Rf value).
Rf = Distance moved by the substance from base line (x) / Distance moved by the solvent from base line (y)
The spots of coloured compounds are visible on TLC plate due to their original colour. The spots of colourless compounds can be detected by putting the plate under ultraviolet light. Another detection technique is to place the plate in a covered jar containing a few crystals of iodine. Spots of compounds, which adsorb iodine, will show up as brown spots. Sometimes an appropriate reagent may also be sprayed on the plate.
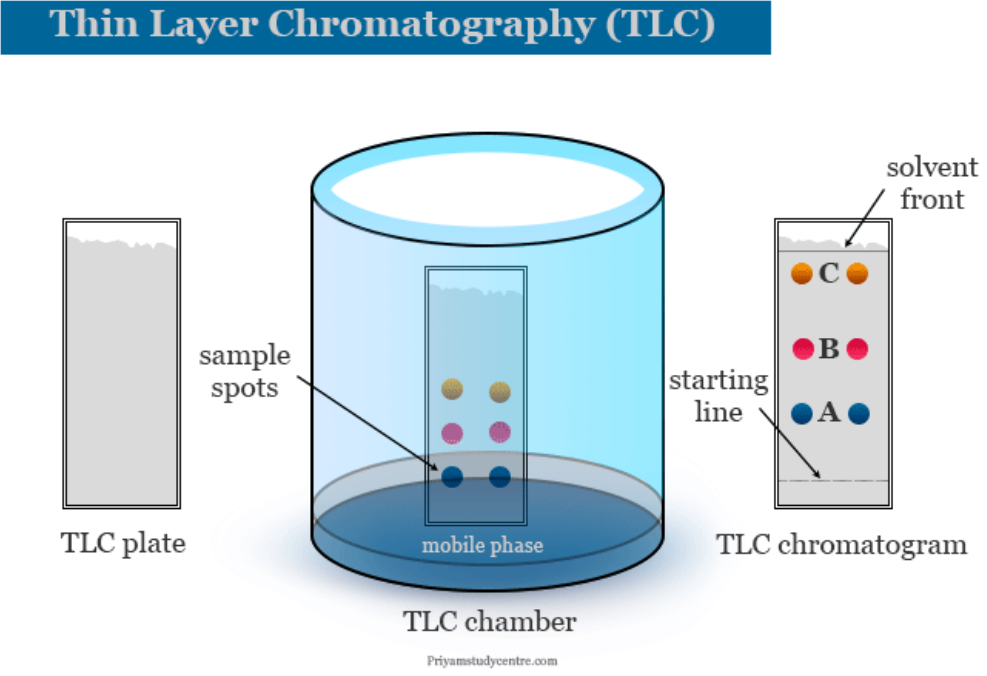
b) Partition Chromatography:
- It is based on continuous differential partitioning of components of a mixture between stationary and mobile phases.
- Paper chromatography is a type of partition chromatography. In paper chromatography, a special quality paper known as chromatography paper is used.
- Chromatography paper contains water trapped in it, which acts as the stationary phase.
- A strip of chromatography paper, spotted at the base with the solution of the mixture, is suspended in a suitable solvent or a mixture of solvents.
- This solvent acts as the mobile phase.
- The solvent rises up the paper by capillary action and flows over the spot.
- The paper selectively retains different components according to their differing partition in the two phases.
- The paper strip is known as a chromatogram.
- The spots of the separated coloured compounds are visible at different heights from the position of initial spot on the chromatogram.
- These spots can be visible by u.v. light or by spraying suitable reagents.
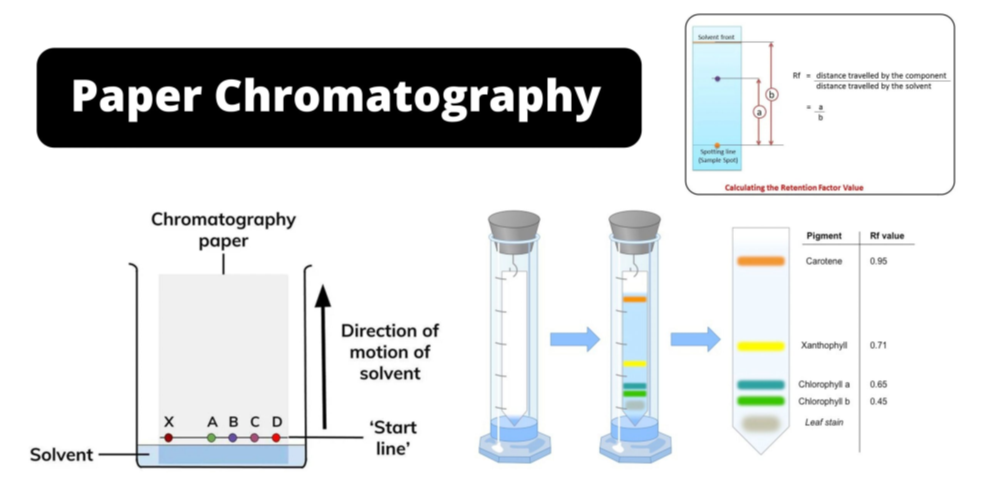
7. Fundamental concepts in organic reaction mechanism
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ORGANIC REACTION MECHANISM-FUNDAMENTAL CONCEPTS
In an organic reaction, the organic molecule (called substrate) reacts with an attacking reagent to form one or more intermediates and finally the products.
Substrate + attacking reagent → Intermediate → Products
A sequential account of different steps in which the reactants are converted to products is called reaction mechanism.
Fission of a covalent bond
A covalent bond can be broken either by homolysis or by heterolysis.
1. Homolysis:
In homolysis or homolytic cleavage, each of the bonded atoms gets one of the electrons of the shared pair. Here the movement of a single electron takes place. The single electron movement is shown by half – headed arrow or fish hook arrow (![]() ).
).
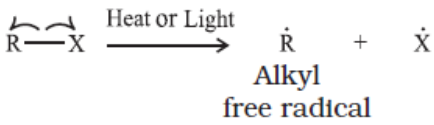
The species formed as a result of homolysis is called free radical. These are species which contain an odd electron or an unpaired electron. There are three types of free radicals – primary (10), secondary (20) and tertiary (30). Their stability increases in the order 10 < 20 < 30.
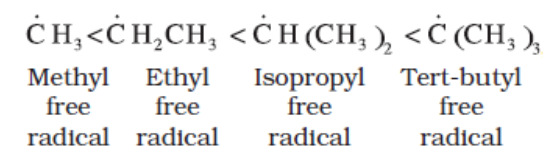
Organic reactions, which take place by homolytic fission are called free radical or homopolar or nonpolar reactions.
2. Heterolysis:
In heterolysis or heterolytic cleavage, the bond breaks in such a manner that the shared pair of electrons remains with one of the parts.
After heterolysis, one atom has a sextet of electron and a positive charge and the other atom has an octet of electron with atleast one lone pair and a negative charge.
For example the bond cleavage in methyl bromide takes place in the following manner.
![]()
A species having a carbon atom possessing sextet of electrons and a positive charge is called a
carbocation (carbonium ion). They are of three types – primary, secondary and tertiary.
Carbocations are highly unstable and reactive species. Their stability increases in the order 10 < 20 < 30. The high stability of tertiary carbocations is due to inductive effect and hyper conjugation. In carbocations, carbon atom is in sp2 hybridisation and hence they have trigonal planar (planar triangular) shape.
If the group attached to the carbon atom is less electronegative than C, due to heterolytic cleavage, a species with C atom containing a shared pair of electrons and negative charge is formed.
![]()
Such a species carrying a negative charge on carbon atom is called carbanion. They are also unstable and reactive. Their stability increases in the order : 30 < 20 < 10.
The organic reactions which proceed through heterolytic bond cleavage are called ionic or heteropolar or polar reactions.
Nucleophiles and Electrophiles
A reagent that brings an electron pair is called a nucleophile (:Nu) and the reaction is called nucleophilic reaction. Or, nucleophiles are electron rich species attack at electron deficient centre. (The word
nucleophile means nucleus seeking).
Example for nucleophiles are OH–, CN–, NO –, Cl–, Br–, I–, H O, NH , R-NH etc.
2 2 3 2
A reagent that takes away an electron pair is called an electrophile (E+) and the reaction is called electrophilic reaction. Or, electrophiles are electron deficient species attack at electron rich centre. (The word electrophile means electron seeking).
Example for electrophiles are carbocations (R+), -CHO, >CO etc.
Electron displacement effects in covalent bonds
In an organic molecule, the electron displacement may take place either under the influence of an atom or in the presence of an attacking reagent. The important types of electron displacement effects are inductive effect, electromeric effect, resonance effect and hyper conjugation.
1. Inductive effect (I effect):
It is a permanent effect arising due to the shifting of sigma electrons through a carbon chain in presence of an atom or group of atom (having different electronegativity) attached to a carbon chain. This effect propagates only through C – C σ bonds. This effect decreases rapidly as the number of C atoms increases.
E.g. 1-chlorobutane CH3 – CH2 – CH2 – CH2 –Cl
Here Cl is more electronegative than C. So the electron pair in the C – Cl bond is shifted towards Cl and it gets a slight –ve charge (δ–) and C gets a slight +ve charge (δ+). This carbon attracts the electron density from the second carbon and so the 2nd carbon gets a relatively smaller positive charge (δδ+).
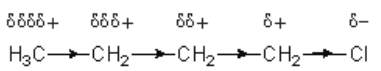
Here Cl atom attracts electron towards it. So we can say that Cl atom has electron withdrawing effect or – I effect (negative inductive effect). So groups which have the ability to attract electron pairs towards it are called – I effect. Example for such groups are –X (F, Cl, Br, I), nitro (-NO2), Cyano (CN–), Carboxy (-COOH), ester (-COOR), aryloxy (-OAr) etc.
Groups which donate electron pairs towards the carbon chain are said to have +I effect or electron donating (releasing) groups. Example for such groups are alkyl groups like methyl (-CH3), ethyl (-CH2-CH3) etc.
2. Electromeric effect (E effect):
![]() It is defined as the complete transfer of a shared pair of π-electrons to one of the atoms joined by a multiple bond in presence of an attacking reagent. It is a temporary effect. It is possible only in compounds containing multiple bonds( alkene or alkyne). This effect cancels when the attacking reagent is removed from the reaction site. The shifting of the electrons is shown by a curved arrow . There are two types of E effects:
It is defined as the complete transfer of a shared pair of π-electrons to one of the atoms joined by a multiple bond in presence of an attacking reagent. It is a temporary effect. It is possible only in compounds containing multiple bonds( alkene or alkyne). This effect cancels when the attacking reagent is removed from the reaction site. The shifting of the electrons is shown by a curved arrow . There are two types of E effects:
a) Positive Electromeric effect (+E effect): Here the pi electrons are transferred to that atom to which the
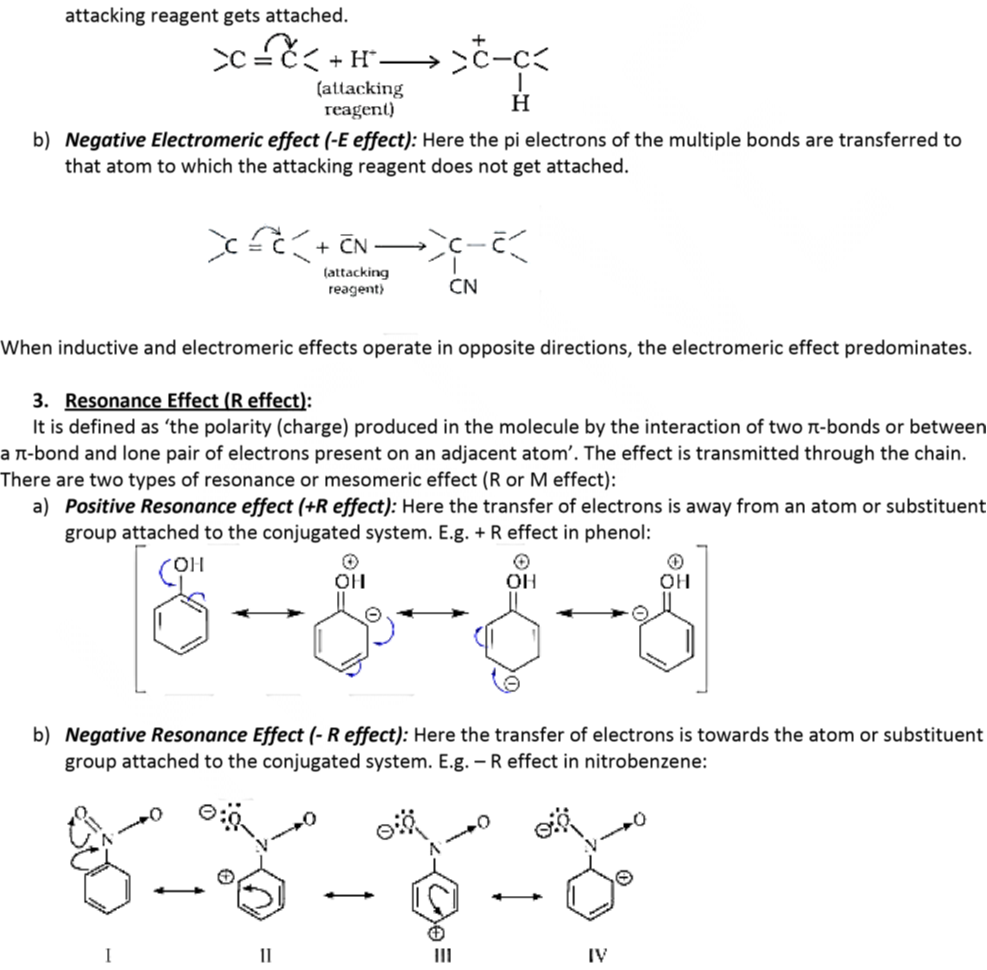
The presence of alternate single and double bonds in an open chain or cyclic system is termed as a conjugated system.
E.g. for +R effect groups: – halogen, –OH, –OR, –OCOR, –NH2, –NHR, –NR2, –NHCOR etc.
E.g. for – R effect groups: – COOH, –CHO, >C=O, – CN, –NO2 etc.
3. Hyper conjugation:
It is a permanent effect. In this effect the σ electrons of C—H bond of the alkyl group enter into partial conjugation with the unsaturated system or with the unshared p orbital. i.e. the σ electrons of C –H bonds get delocalised.
e.g. ethyl cation (CH3-CH +)
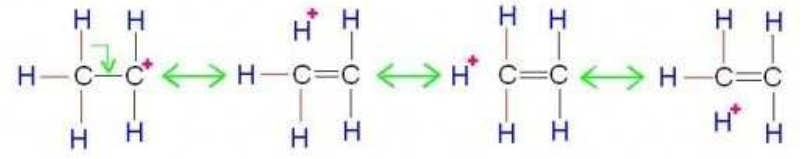
Hyper conjugation stabilises the carbocation because electron density from the adjacent σ bond helps in dispersing the positive charge. In general, the greater the number of alkyl groups attached to a positively charged carbon atom, the greater is the hyper conjugation interaction and stabilisation of the cation.
Thus the relative stability of carbocations is in the order: (CH3)3C+ > (CH3)2CH+ > CH3-CH2+ . CH3+.
Here tertiary carbocation has 9, isopropyl has 6, ethyl carbocation has 3 and methyl carbocation has zero hyper conjugative structures.
Hyper conjugation is also called no-bond resonance and it is also possible in alkenes and alkyl arenes.
Types of Organic reactions
Organic reactions can be classified into the following categories:
-
- Substitution reactions
- Addition reactions
- Elimination reactions
- Rearrangement reactions
6. Isomerism
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ISOMERISM
The phenomenon of existence of two or more compounds having the same molecular formula but different structural formula or spatial arrangement of atoms is known as isomerism. Such compounds are called as isomers. Isomers have different physical and chemical properties. Isomerism can be broadly classified into two – structural isomerism and stereo isomerism.
1. Structural isomerism
Compounds having same molecular formula but different structural formula (arrangement of atoms) are called structural isomers and the phenomenon is called structural isomerism. There are mainly four types of structural isomerism:
- Chain Isomerism: Isomers differ in carbon chain or skeleton are called chain isomers and the phenomenon is called chain isomerism.
E.g.: Pentane(C5H12)
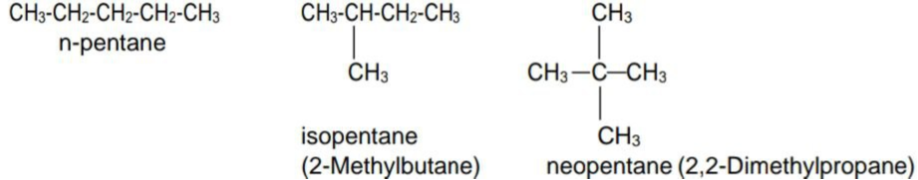
- Position isomerism: Isomers which differ in the position of the substituent or side chain are called position isomers and the phenomenon is called position isomerism.
E.g. : Alcohol with molecular formula C4H10O may be 1-butanol or 2 butanol
![]()
- Functional group isomerism: Isomers which differ in the functional group are called functional group isomers and the phenomenon is called functional group isomerism. This isomerism is shown by alcohols and ethers and aldehydes and ketones.
E.g. compound with the molecular formula C2H6O may be an alcohol ethanol (CH3-CH2OH) or an ether methoxy methane (CH3-O-CH3).
- Metamerism: Isomers which differ in the carbon chain (alkyl groups) around the functional group are called metamers and the phenomenon is called metamerism. It is commonly shown by ethers.
E.g.: Ether with molecular formula C5H12O may be methoxybutane (CH3-O-CH2-CH2-CH2-CH3) or ethoxypropane (CH3-CH2-O-CH2-CH2-CH3).
2. Stereo isomerism
Compounds having same molecular formula but different spatial arrangement of atoms are called stereoisomers and the phenomenon is called stereoisomerism. They have same atom to atom bond.
There are two types of stereo isomerism – Geometrical isomerism and Optical isomerism. The diagrammatic representation of different types of isomerism is:
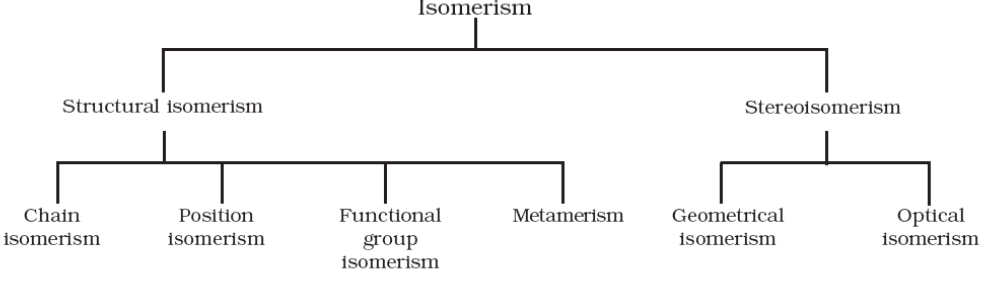
5. Nomenclature of organic compounds
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
NOMENCLATURE OF ORGANIC COMPOUNDS
An organic compound has two types of names – Common name and IUPAC name. The common name is based on the source or some properties. For e.g. citric acid is named so because it is found in citrus fruits and the acid found in red ant is named formic acid since the Latin word for ant is formica.
IUPAC Nomenclature of organic compounds
A systematic name of an organic compound is generally derived by identifying the parent hydrocarbon and the functional group(s) attached to it. This name is called IUPAC name. It contains two parts – word root and suffix or prefix. The word root indicates the number of carbon atoms in the compound. The word roots for compounds containing 1 -12 carbon atoms are as follows:
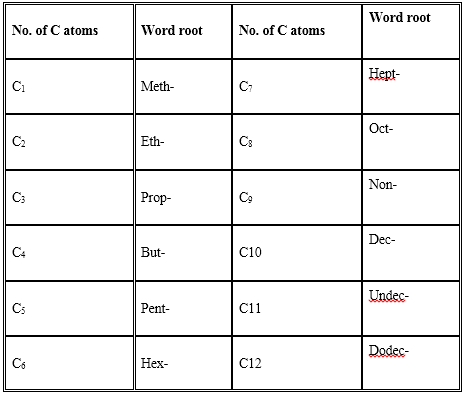
There are two types of suffixes – primary suffix and secondary suffix. Primary suffix indicates saturation or unsaturation [for alkane the primary suffix is –ane, alkene –ene and for alkyne –yne]. Secondary suffix indicates the type of functional group. Some functional groups are also indicated as prefixes.
Nomenclature of branched chain alkanes:
A branch (side chain or substituent) is obtained by removing a hydrogen atom from an alkane. The resulting group is called an alkyl group [alkane – H = alkyl (i.e. word root + yl)]. The names of some common branches are as follows:
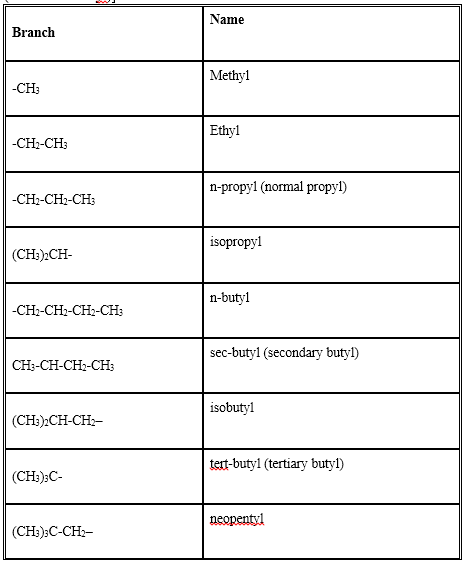
Rules for naming branched chain alkanes:
IUPAC recommenced the following rules for naming a branched chain alkane.
- Select the longest continuous chain of carbon atoms. This chain is called parent chain or root chain. If there is more than one such chain, the chain that contains maximum number of branches is selected as the parent chain. Also identify all the branches or substituents.
- Number the carbon atoms of the parent chain in such a way that the branched carbon atoms get the lowest possible numbers.
- The names of alkyl groups attached as branches are then prefixed to the name of the parent alkane and position of the substituents is indicated by the appropriate numbers.
- If different alkyl groups are present, they are listed in alphabetical order. In alphabetical order, the prefixes iso- and neo- are considered to be the part of the fundamental name of alkyl group. The prefixes sec- and tert- are not considered to be the part of the fundamental name.
- If two or more identical substituent groups are present then their numbers are indicated by prefixes like di (for 2), tri (for 3), tetra (for 4), penta (for 5) etc and the numbers are separated by commas. The number and word are separated by a hyphen. (The IUPAC name is written as a single word).
For example:
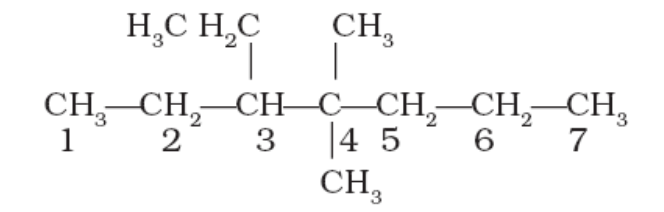
3-Ethyl-4,4-dimethylheptane
6. If the two substituents are found in equivalent positions, the lower number is given to the one coming first in the alphabetical listing. For example:
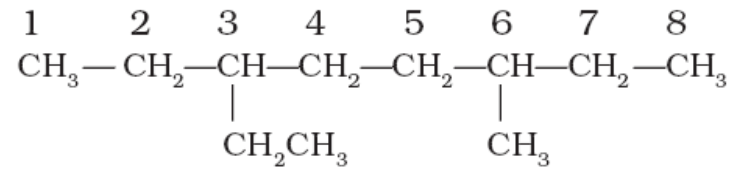
The above compound is 3-ethyl-6-methyloctane and not 6-ethyl-3-methyloctane.
7. While naming the branched alkyl groups, the carbon atom of the branch that attaches to the root alkane is numbered 1.
For example:
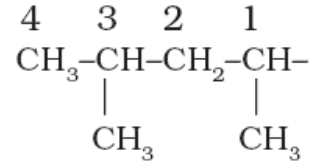
1,3-dimethyl butyl-
IUPAC nomenclature of compounds containing functional groups
For naming organic compounds containing functional group, the following rules are used:
- Select the longest continuous chain containing the functional group.
- Number the carbon atoms in such a way that the carbon to which the functional group is attached should get the lowest possible number. In the case of functional groups containing carbon atom like –CHO, -CN, – COOH, -CONH2, -COX. -COOR etc. the numbering should start from the carbon atom of the functional group. (i.e. carbon atom of these groups should be numbered as 1). (But for ketones, the functional group –CO- should get the lowest possible number).
- The name of the functional group is indicated by the following suffix or prefix.
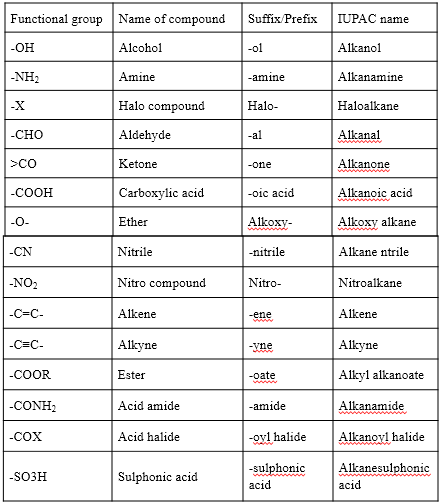
In the case of suffixes, the ending –e of the corresponding alkane is replaced. E.g. IUPAC name of the alcohol CH3-OH is methanol (methane + ol). But for nitriles, the –e of the corresponding alkane is retained.
E.g. IUPAC name of CH3-CH2-CN is propanenitrile.
In the case of alkenes and alkynes, the suffix –ane of the alkane is replaced by –ene and –yne respectively. (i.e. word root + ene or yne). For naming alkenes or alkynes, the numbering is done in such a way that the double or triple bond should get the lowest possible number.
Some examples are:
Nomenclature of organic compounds containing more than one functional groups (Poly functional compounds)
Here one of the functional groups is chosen as the principal functional group and the compound is named on that basis. The remaining functional groups (called subordinate functional groups) are named as substituents using the appropriate prefixes. The choice of principal functional group is made on the basis of order of preference. The order of decreasing priority for some functional groups is:
-COOH, –SO3H, -COOR (R=alkyl group), -COCl, -CONH2, -CN,-CHO, >CO, -OH, -NH2, >C=C<, -C≡C-
The groups like alkyl (–R), phenyl (C6H5-), halogens (F, Cl, Br, I), nitro (–NO2), alkoxy (–OR) etc. are always prefix substituents.
For example if a compound contains both alcoholic and aldehydic groups, it is named as hydroxyalkanal, since here aldehydic group is the principal functional group and –OH group is the subordinate functional group. The prefix names of some functional groups are as follows:
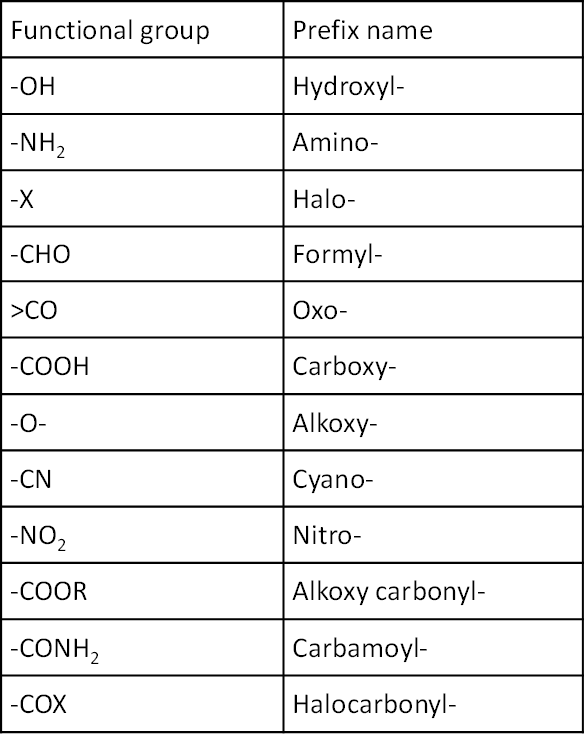
While numbering the carbon chain, the principal functional group should get the lowest possible number. Some examples are
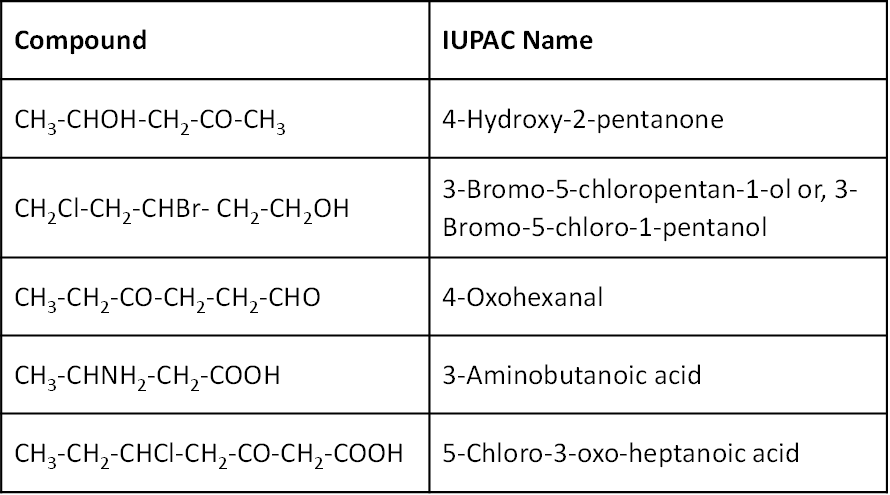
If a compound contains more than one same functional group, their number is indicated by adding the numeral prefixes di, tri, etc. before the suffix. In such cases the full name of the parent alkane is written before the suffix. However, the ending – ne of the parent alkane is dropped in the case of compounds having more than one double or triple bonds.
When both double and triple bonds are present, the double bonds are given the lowest numbers. Here first give the suffix of the double bond (-en) and then that of the triple bond (-yne) [the ending –e of the suffix –ene is avoided].
Examples:
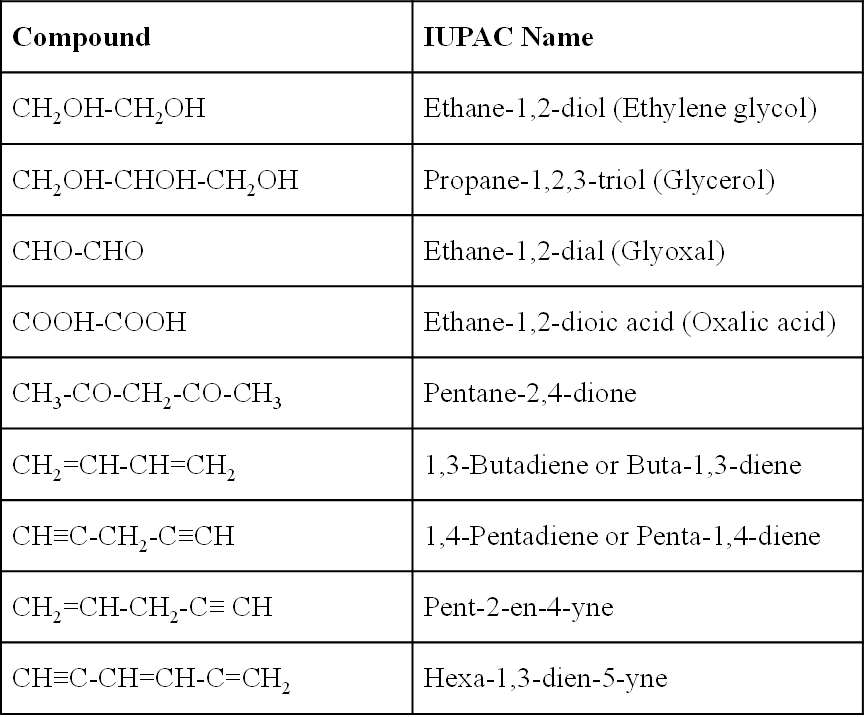
(The names given in the brackette are the common names)
Nomenclature of Substituted Benzene Compounds
For IUPAC nomenclature of substituted benzene compounds, the substituent is placed as prefix to the word benzene. But common names of some compounds are accepted by IUPAC.
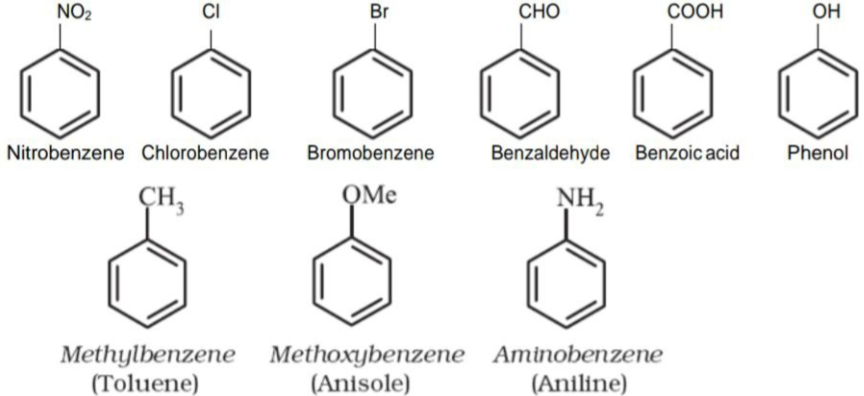
Nomenclatrue of di or polysubstituted benzene
If benzene ring is disubstituted, the position of substituents is indicated by numbering the carbon atoms of the ring such that the substituents get the lowest possible numbers.
Example – Dibromobenzene
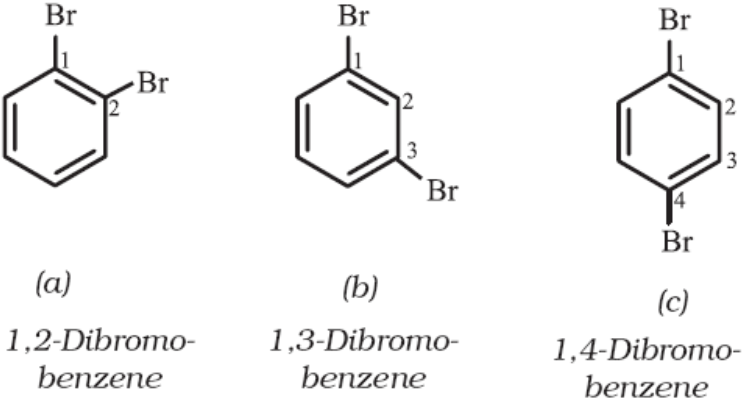
In the common system of nomenclature the terms ortho (o), meta (m) and para (p) are used as prefixes to indicate the relative positions 1,2- 1,3- and 1,4- respectively. So 1,2-dibromobenzene is named as ortho (or just o-) dibromobenzene, 1,3-dibromobenzene as meta (or just m-) dibromobenzene and 1,4-dibromobenzene as para (or just p-)-dibromobenzene.
For tri – or higher substituted benzene derivatives, these prefixes cannot be used and the compounds are named by identifying substituent positions on the ring by following the lowest locant rule. In some cases, common name of benzene derivatives is taken as the base compound. Substituent of the base compound is assigned number1 and then the direction of numbering is chosen such that the next substituent gets the lowest number. The substituents are named in alphabetical order.
Some examples are:
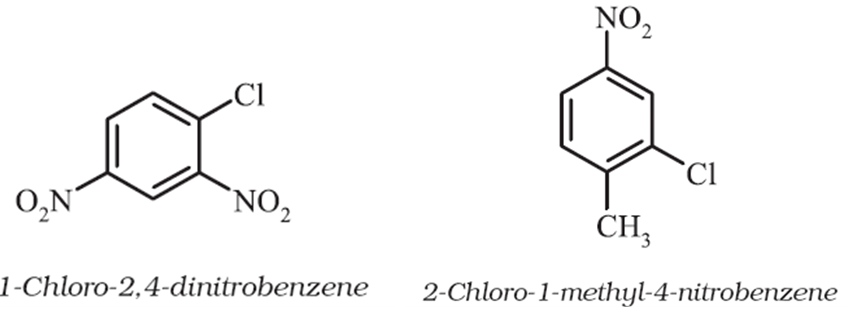
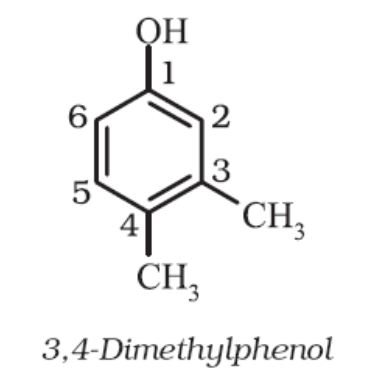
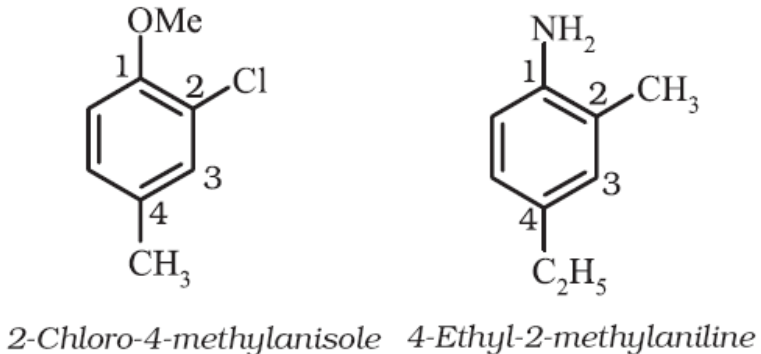
When a benzene ring is attached to an alkane with a functional group, it is considered as substituent, instead of a parent. The name for benzene as substituent is phenyl (C6H5-, also abbreviated as Ph).
Example:
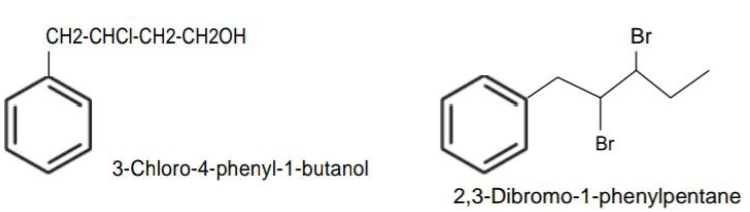
9. Qualitative analysis of organic compounds
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
QUALITATIVE ANALYSIS OF ORGANIC COMPOUNDS
An organic compound mainly contains carbon and hydrogen. Some compounds may also contain oxygen, nitrogen, sulphur, halogens and phosphorus.
Detection of Carbon and Hydrogen
Organic compound is heated with copper (II) oxide [CuO]. Carbon present in the compound is oxidised to carbon dioxide and hydrogen to water. CO2 can be tested by passing through lime-water, which turns milky and water can be tested with anhydrous copper sulphate, which turns blue.
C + 2CuO ⎯⎯Δ → 2Cu + CO2
2H + CuO ⎯⎯Δ → Cu + H2O
CO2 + Ca(OH)2 ⎯→ CaCO3↓ + H2O
Detection of Nitrogen, Sulphur and Halogens
Nitrogen, sulphur and halogens present in an organic compound are detected by “Lassaigne’s test”. Here the organic compound is fused with metallic sodium in a fusion tube. It is then plunged into distilled water taken in a china dish. The solution is boiled and filtered. The filtrate is known as sodium fusion extract.
Principle: In an organic compound, nitrogen, sulphur and halogen atoms are present in covalent form. By heating with metallic sodium, these elements are converted to ionic form as follows:
Na + C + N ⎯⎯Δ → NaCN
2Na + S ⎯⎯Δ → Na2S
Na + X ⎯⎯Δ → Na X (X = Cl, Br or I)
For the detection of the elements, the following tests are done:
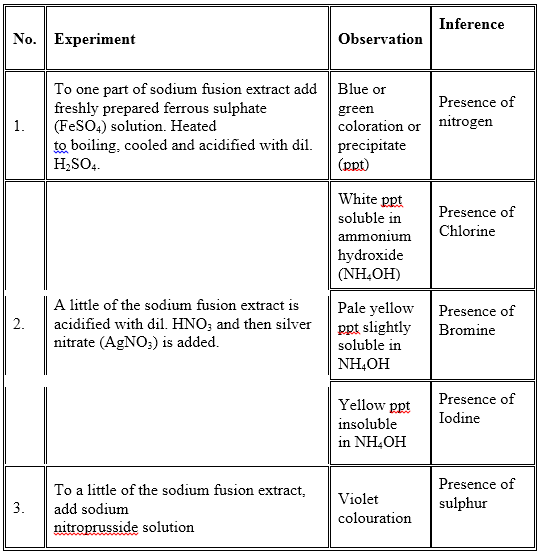
Test for Phosphorus
The organic compound is heated with an oxidising agent like sodium peroxide. The phosphorus present in the compound is oxidised to phosphate. The solution is boiled with nitric acid and then treated with ammonium molybdate. A yellow colouration or precipitate indicates the presence of phosphorus.
10. Quantitative analysis
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
QUANTITATIVE ANALYSIS OF ORGANIC COMPOUNDS
The percentage composition of elements present in an organic compound is determined by the following methods:
1. Estimation of Carbon and Hydrogen
Carbon and hydrogen are estimated by Liebig’s combustion method. In this method, a known mass of an organic compound is burnt in the presence of excess of oxygen and copper(II) oxide. Then carbon is oxidised to CO2 and hydrogen is oxidised to H2O.
CxHy + (x + y/4) O2 ⎯→ x CO2 + (y/2) H2O
The water so produced is absorbed in a weighed U-tube containing anhydrous calcium chloride and carbon dioxide is absorbed in another U-tube containing concentrated solution of potassium hydroxide. These tubes are connected in series. The increase in masses of calcium chloride and potassium hydroxide gives the amounts of water and carbon dioxide from which the percentages of carbon and hydrogen are calculated.
Calculations: Let the mass of organic compound be m g, mass of water and carbon dioxide produced be m1 and m2 g respectively.
Percentage of hydrogen = 2 x m1 x 100 / 18 x m
Percentage of carbon = 12 x m2 x 100 % / 44 x m
2. Estimation of Nitrogen
There are two methods for estimation of nitrogen: (i) Dumas method and (ii) Kjeldahl’s method.
(i) Dumas method:
Here the organic compound is heated with copper oxide in an atmosphere of carbon dioxide so that free nitrogen, carbon dioxide and water are produced.
CxHyNz + (2x + y/2) CuO ⎯→ x CO2 + y/2 H2O + z/2 N2 + (2x + y/2) Cu
This mixture of gases is collected over an aqueous solution of potassium hydroxide which absorbs carbon dioxide. Nitrogen is collected in the upper part of the graduated tube
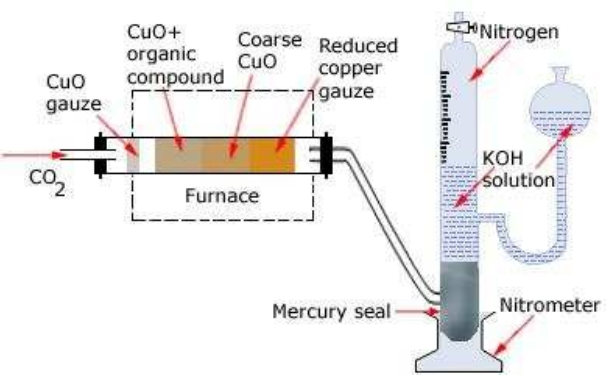
Calculations:
Let the mass of organic compound = m g Volume of nitrogen collected = V1 mL Room temperature = T1 K
Volume of nitrogen at STP = P1V1 x 273 / 760 × T1 = V mL
Where P1 and V1 are the pressure and volume of nitrogen gas.
P1= Atmospheric pressure – Aqueous tension
We know that 22400 mL N2 at STP weighs 28 g. Therefore, VmL N2 at STP weighs = 28 x V / 22400 g
Percentage of nitrogen = 28 x V x 100 % / 22400 x m
(ii) Kjeldahl’s method:
Here the organic compound containing nitrogen is heated with concentrated sulphuric acid. Nitrogen in the compound gets converted to ammonium sulphate. The resulting acid mixture is then heated with excess of sodium hydroxide. The liberated ammonia gas is absorbed in an excess of standard solution of sulphuric acid. The amount of ammonia produced is determined by estimating the amount of sulphuric acid consumed in the reaction. It is done by estimating unreacted sulphuric acid left after the absorption of ammonia by titrating it with standard alkali solution. The difference between the initial amount of acid taken and that left after the reaction gives the amount of acid reacted with ammonia.
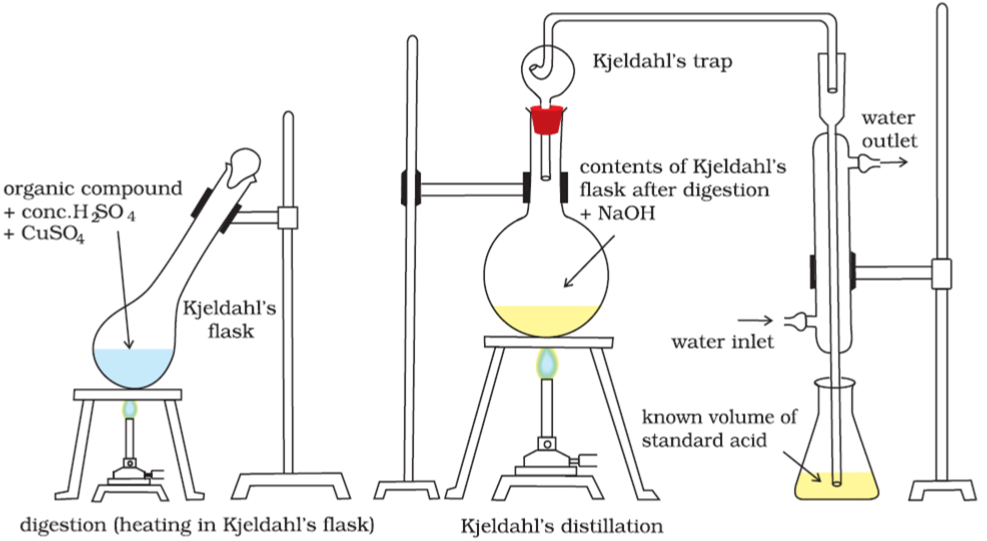
Organic compound + H2SO4 ⎯→ (NH4)2SO4
2 NaOH Na2SO4 + NH3 + H2O 2NH3 + H2SO4 ⎯→ (NH4)2SO4
Calculations:
Let the mass of organic compound taken = m g Volume of H2SO4 of molarity M, taken = V ml
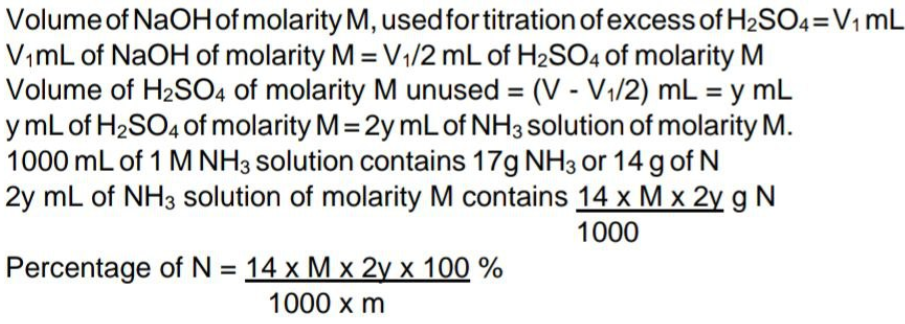
Note: Kjeldahl’s method is not applicable to compounds containing nitrogen in nitro and azo groups and nitrogen present in the ring (e.g. pyridine) as nitrogen of these compounds does not change to ammonium sulphate under these conditions.
3. Estimation of halogens (Carius method):
Here a known mass of an organic compound is heated with fuming nitric acid in the presence of silver nitrate contained in a hard glass tube known as Carius tube, in a furnace. Carbon and hydrogen present in the compound are oxidised to carbon dioxide and water. The halogen present forms the corresponding silver halide (AgX). It is filtered, washed, dried and weighed.
Calculations:
Let the mass of organic compound taken = m g
Mass of AgX formed = m1 g
1 mol of AgX contains 1 mol of halogen
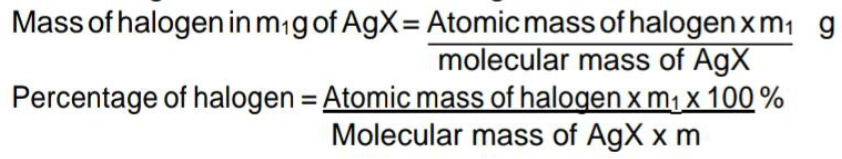
4. Estimation of Sulphur (Carius method):
A known mass of an organic compound is heated in a Carius tube with sodium peroxide or fuming nitric acid. Sulphur present in the compound is oxidised to sulphuric acid. It is precipitated as barium sulphate by adding excess of barium chloride solution. The precipitate is filtered, washed, dried and weighed. The percentage of sulphur can be calculated from the mass of barium sulphate (BaSO4).
Calculations:
Let the mass of organic compound taken = m g and the mass of barium sulphate formed = m1 g 1 mol of BaSO4 = 233 g BaSO4 = 32 g sulphur
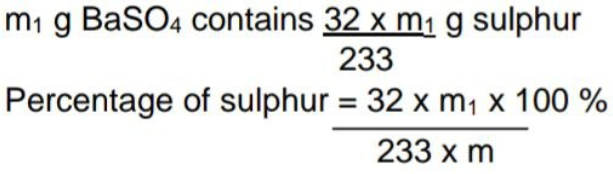
5. Estimation of Phosphorus
A known mass of an organic compound is heated with fuming nitric acid. Phosphorus present in the compound is oxidised to phosphoric acid. It is precipitated as ammonium phosphomolybdate [(NH4)3PO4.12MoO3] by adding ammonia and ammonium molybdate.
Calculations:
Let the mass of organic compound taken = m g and mass of ammonium phosphomolydate = m1g
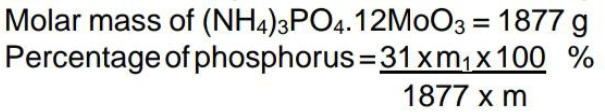
6. Estimation of Oxygen
The percentage of oxygen in an organic compound is usually found by difference between the total percentage composition (100) and the sum of the percentages of all other elements.
i.e. percentage of oxygen = 100 – sum of the percentage of all the other elements.
2. Tetravalence of carbon : Shapes of organic compounds
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
TETRAVALENCE OF CARBON: SHAPES OF ORGANIC COMPOUNDS
Carbon is a unique element and it can form a large number of compounds due to the following reasons:
1. Tetravalency of carbon: In all of its compounds, the valency of carbon is four. Carbon has 4 electrons in its valency shell and requires 4 more electrons to complete the octet. So it attains the octet configuration by forming 4 covalent bonds.
2. Ability to form single bond and multiple bonds: C can form single bond and multiple bond (double or triple bond) with itself and also with other elements like oxygen, nitrogen etc. This is possible by sp3, sp2 or sp hybridisation.
3. Catenation: Carbon shows catenation. It is the self linking property of an element to form long chains and rings.
4. Isomerism: Carbon compounds can show isomerism. It is the phenomenon in which compounds having same molecular formula but different structural formula or spatial arrangement of atoms.
3. Structural representations of organic compounds
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
STRUCTURAL REPRESENTATION OF ORGANIC COMPOUNDS
An organic compound can be represented by the following ways:
Complete structural formula:
Here all the bonds between atoms are denoted by dashes ( ). A single dash represents a single bond, a double dash represents a double bond and a triple dash represents a triple bond. E.g.
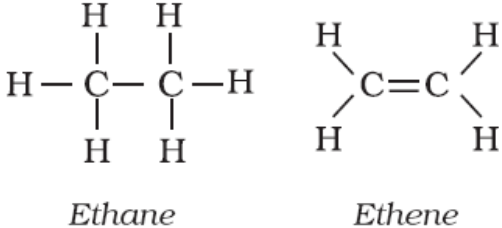
Condensed stuctural formula: Here the carbon-hydrogen bonds or all the bonds are omitted except the multiple bonds. It is a simplified representation of an organic compound.
E.g. ethane – CH3CH3, propane – CH3CH2CH3, butane – CH3CH2CH2CH3, ethene – CH2=CH2 etc.
The condensed formula can again simplified as follows:
Butane – CH3(CH2)2CH3, Hexane- CH3(CH2)4CH3, Decane – CH3(CH2)8CH3 etc.
Bond line representation: It is the simplest form of representation of an organic compound. Here carbon and hydrogen atoms are not shown and the lines representing carbon-carbon bonds are drawn in a zig-zag fashion. The only atoms specifically written are oxygen, chlorine, nitrogen etc. The free terminals denote methyl (–CH3) groups.
Three-Dimensional Representation (Wedge Representation): Here the structure of an organic molecule can be represented by using solid (![]() ) and dashed (
) and dashed ( ![]() ) wedges.
) wedges.
1. The solid-wedge is used to indicate a bond projecting out of the plane of paper, towards the observer.
2. The dashed-wedge indicates the bond projecting out of the plane of the paper and away from the observer.
3. The broad end of the wedge is always towards the observer. The bonds lying in plane of the paper are depicted by using a normal line (—).
E.g. methane
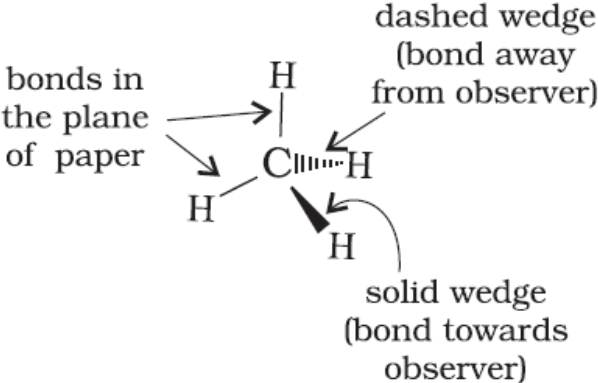
4. Classification of organic compounds
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
CLASSIFICATION OF ORGANIC COMPOUNDS
Organic compounds can be broadly classified into two – Acyclic or open chain compounds and cyclic or ring compounds.
1. Acyclic or open chain or aliphatic compounds: In these compounds, the carbon atoms are joined together to form long chains which may be straight chain or branched chain.
(i) They are further classified as saturated compounds and unsaturated compounds.
(ii) Saturated compounds contain only carbon – carbon single bonds. But unsaturated compounds contain atleast one carbon – carbon multiple bond (double or triple bond.
(iii) Saturated hydrocarbons are called alkanes and unsaturated hydrocarbons are of two types – alkenes and alkynes.
2. Cyclic or closed chain or ring compounds: In these compounds, the carbon atoms are joined together to form rings. These rings may be homocyclic or heterocyclic.
-
- If the ring contains only carbon atoms, it is called homocyclic compound and if it contains atoms other than carbon (like O, N, S etc), it is called heterocyclic compound.
- Homocyclic compounds are further classified into two – Alicyclic compounds and Aromatic compounds.
- Alicyclic compounds contain atleast one carbo-cyclic ring. Alicyclic hydrocarbons are of three types – cycloalkanes, cycloalkenes and cycloalkynes.
- Aromatic compounds are some special type of compounds.
- These are of two types. Aromatic compounds containing benzene ring are called benzenoid compounds and those which do not contain benzene ring are called non-benzenoid compounds.
- E.g. for a non-benzenoid aromatic compound is tropolone. Heterocyclic compounds may be alicyclic heterocyclic compounds or aromatic heterocyclic compounds.
The classification of organic compounds can be diagrammatically represented as follows:
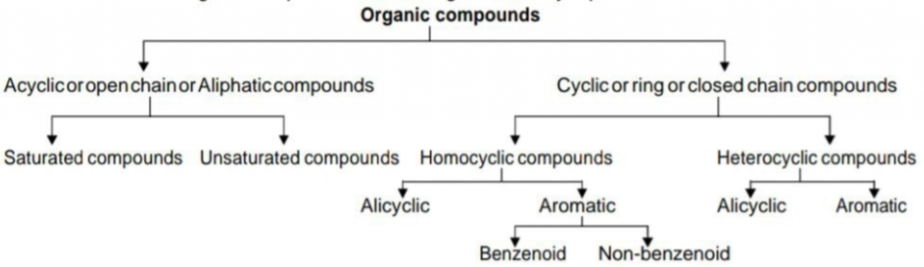
Functional groups: Atoms or group of atoms (except hydrogen) which are bonded to carbon atoms are called functional groups. These groups are responsible for the characteristic chemical properties of the organic compounds. Some important functional groups, their names and name of the compounds are listed below
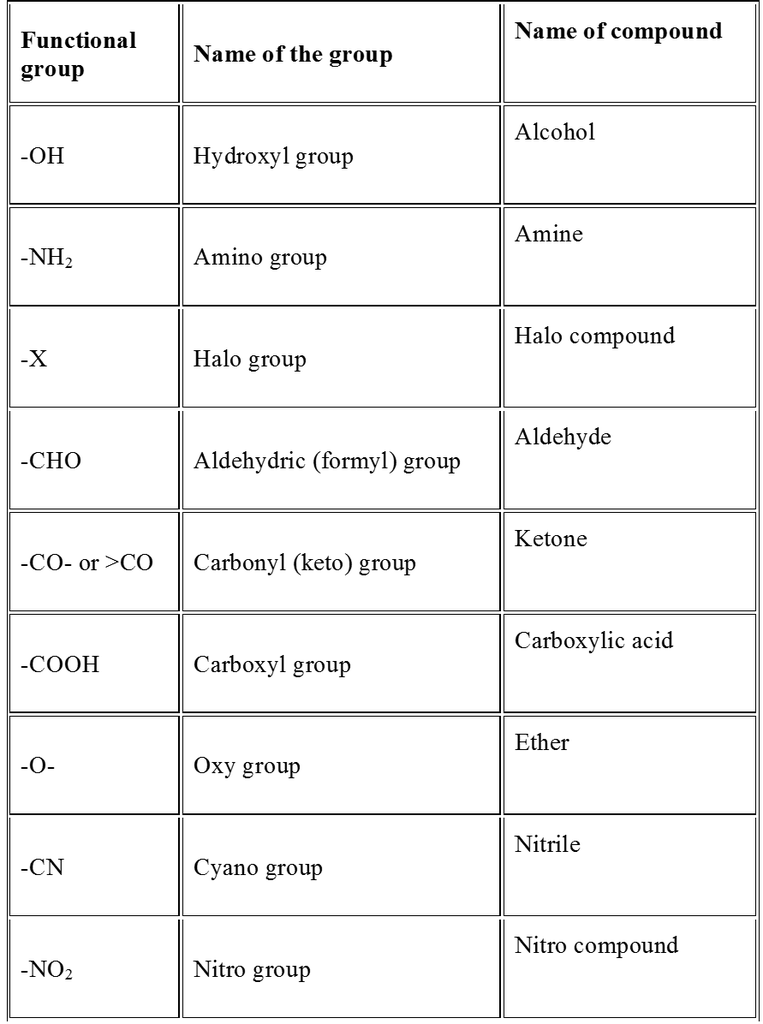
Homologous series: A series or group of organic compounds in which adjacent members are differed by a –CH2 group is called a homologous series. The members of a homologous series are called homologues. They contain same functional groups, have similar chemical properties and show gradation in physical properties. They can be prepared by some general methods of preparation. E.g. for homologous series are alkanes, alkenes, alkynes, alcohols, ethers, carboxylic acids, aldehydes, ketones, amines, halo compounds etc.
1. Classification
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
CHAPTER- 13 HYDROCARBONS
CLASSIFICATION
Organic compounds containing carbon and hydrogen atoms only are called hydrocarbons. Depending on the types of C-C bond, they can be classified into three – saturated, unsaturated and aromatic hydrocarbons. Saturated hydrocarbons are also called alkanes. They contain only C-C single bonds. Unsaturated hydrocarbons contain atleast one carbon-carbon double bond (alkene) or carbon-carbon triple bond (alkyne). Aromatic hydrocarbons are a special type of cyclic compounds. They are also called arenes.
1. Environmental pollution
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
CHAPTER- 14 ENVIRONMENTAL CHEMISTRY
ENVIRONMENTAL POLLUTION
• Environmental Chemistry
It is the branch of science which deals with the chemical changes in the environment. It includes our surroundings as air, water, soil, forest etc.
It is the effect of undesirable changes in our surroundings that have harmful effects on plants, animals and human beings.
• Pollutants
A substance, which causes pollution, is known as pollutant. Pollutants can be solid, liquid or gaseous substances. Present in higher concentration, it can be produced due to human activities or natural happenings.
2. Alkanes
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ALKANES
Alkanes are saturated open chain hydrocarbons containing carbon-carbon single bonds. They form a homologous
series. Their general molecular formula is CnH2n+2. In alkanes, all the C atoms are sp3 hybridised. So each C atom has a regular tetrahedral shape.
Alkanes do not react with acids, bases and other reagents under normal conditions. So they are also called paraffins. (In Latin paraffin means little affinity).
Preparation of alkanes
1. From unsaturated hydrocarbons: Alkenes and alkynes add Hydrogen in presence of finely divided catalysts like Ni, Pd or Pt to form alkanes. This process is called hydrogenation.
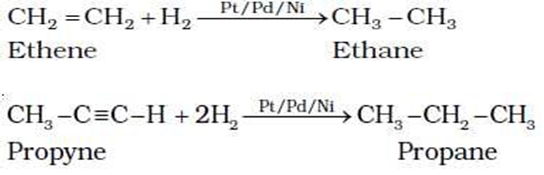
2. From alkyl halides:
a) Alkyl halides on reduction with zinc and dil. HCl, we get alkanes
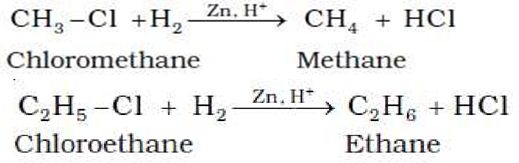
b) Wurtz reaction:
Alkyl halides react with metallic sodium in dry ether to form alkanes. This reaction is known as Wurtz reaction. The alkane formed contains double the number of C atoms than that present in the alkyl halide. So this method is used for the preparation of alkanes with even number of carbon atoms.
R-X + 2Na + X-R → R-R + 2NaX
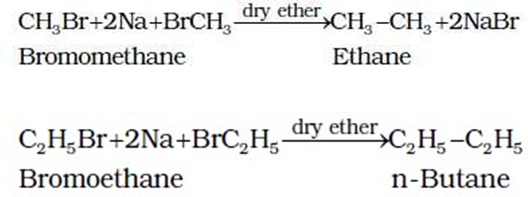
When two different alkyl halides are used, we get a mixture of alkanes.
3. From carboxylic acids:
a) Decarboxylation: Sodium salt of carboxylic acids (R-COONa) on heating with soda lime (a mixture of NaOH
and CaO), we get an alkane containing one carbon atom less than that of the carboxylic acid. This process is known as decarboxylation, since it involves the elimination of a CO2 molecule from the carboxylic acid.
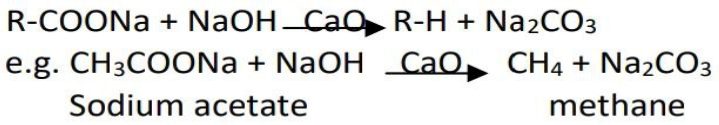
b) Kolbe’s Electrolytic method: An aqueous solution of sodium or potassium salt of a carboxylic acid on electrolysis gives alkane containing even number of carbon atoms.
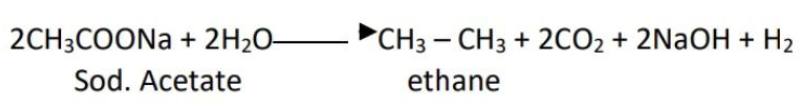
Physical Properties
- Boiling point of alkanes increase with increase of molecular mass (or with number of C atoms).
- This is because in alkanes there is only a weak van der Waal’s force of attraction between different molecules.
- As the molecular size increases, the surface area increases and hence van der Waal’s force increases.
- So the boiling point increases.
- The b.p of isomeric alkanes decreases with branching.
- As the branching increases, the molecule attains the shape of a sphere.
- So the surface area decreases and hence the b.p.
Chemical Properties
1. Substitution reaction
These are reactions in which hydrogen atom of an alkane is replaced by other atoms or atom groups.
E.g. when an alkane is treated with halogen in the presence of diffused sunlight or uv light, we get haloalkane. This reaction is known as halogenations.
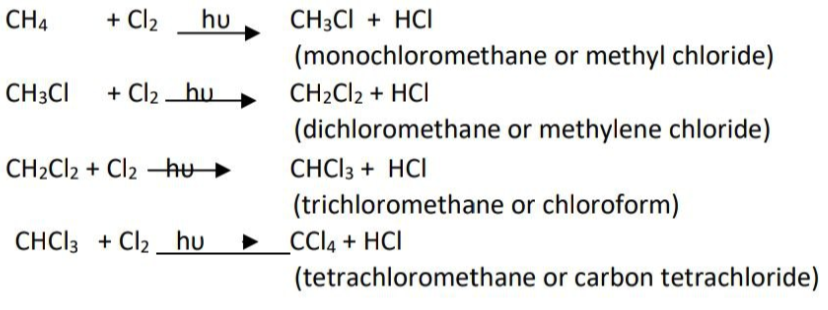
Mechanism
Halogenations takes place by free radical chain mechanism and it involves three steps – initiation, propagation and termination.
i) Initiation step: The reaction is initiated by the homolysis of chlorine molecule in presence of sunlight.

ii) Propagation step: The chlorine free radical attacks the methane molecule and form methyl free radical and HCl.
![]()
The methyl radical then attacks the second Cl2 molecule to form CH3Cl and Chlorine free radical.
![]()
The above two steps repeat and thus the reaction propagates.
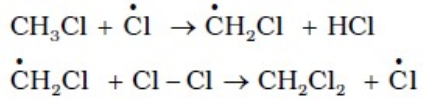
iii) Termination step: The reaction stops after some time due to any one of the following reactions:
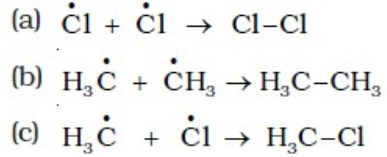
2. Combustion (Oxidation):
On combustion in presence of air or oxygen, alkanes give CO2, H2O and large amount of heat.
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) + heat
The general combustion equation for any alkane is:
CnH2n+2 + (3n+1)/2 O2 → nCO2 + (n+1) H2O
Incomplete combustion of alkanes with insufficient amount of air or O2 gives carbon black.
3. Controlled Oxidation:
Alkanes on heating with O2 at high pressure and in presence of suitable catalysts to form different compounds
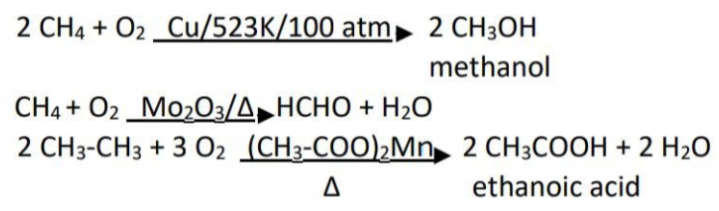
4. Isomerisation: n-Alkanes on heating in the presence of anhydrous aluminium chloride and hydrogen chloride gas isomerise to branched chain alkanes.
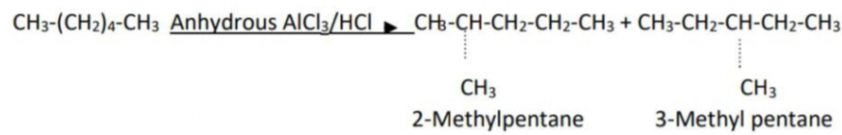
Aromatization: n-Alkanes having six or more carbon atoms on heating to 773K at 10-20 atmospheric pressure in the presence of oxides of vanadium, molybdenum or chromium supported over alumina, we get aromatic compounds. This reaction is known as aromatization or reforming.
5. Pyrolysis: Alkanes having six or more carbon atoms on heating at higher temperature decompose to form lower alkanes, alkenes etc. This reaction is known as pyrolysis.
Conformations of Alkanes
- Alkanes contain carbon-carbon sigma (σ) bonds.
- Since, electron distribution of the sigma bonds is symmetrical around the bond axis, rotation around C–C bond is allowed.
- This rotation changes the spatial arrangements of atoms attached to C atoms.
- These different spatial arrangements of atoms arising due to free rotation around a C-C single bond are called conformations or conformers or rotamers.
Conformations of ethane
- Ethane contains a C-C σ bond and each carbon atom contains three hydrogen atoms. Due to free rotation of C atoms around the single bond, the spatial arrangement of hydrogen atoms attached to the C atoms change. Thus ethane can show an infinite number of conformational isomers.
- If we fix one carbon atom and rotate the other, there arise two extreme cases called eclipsed and staggered conformations.
- In eclipsed conformation, the hydrogen atoms attached to each carbon atoms are closed together as possible. Or, here the hydrogen atoms of the 2nd carbon atoms are exactly behind that of the first.
- In staggered conformation, the hydrogen atoms are far apart as possible. Any conformations between eclipsed and staggered conformations are called skew conformations.
- Staggered conformation is stabler than eclipsed form. This is because in staggered form, the electron clouds of carbon-hydrogen bonds are very far apart.
- So there is minimum repulsive forces, minimum energy and maximum stability. But in eclipsed form, the electron clouds are close to each other. So the repulsion is maximum and the stability is minimum.
Eclipsed and staggered conformations can be represented by Sawhorse and Newman projection formulas.
1. Sawhorse projections:
- Here the molecule is viewed along the molecular axis. C–C bond is denoted by a longer straight line.
- The front carbon is shown at the lower end of the line and the back carbon is shown at the upper end.
- Each carbon has three lines attached to it corresponding to three hydrogen atoms.
Sawhorse projections of eclipsed and staggered conformations of ethane are as follows:
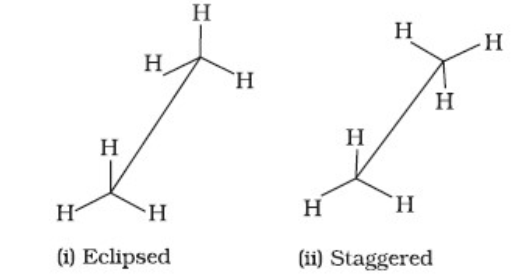
2. Newman projections:
- Here the molecule is viewed at the C–C bond head on (i.e. from the front side).
- The front carbon atom is represented by a point.
- Three hydrogen atoms attached to this carbon atom are shown by three lines drawn at an angle of 120° to each other.
- The back carbon atom is represented by a circle and the three hydrogen atoms are shown attached to it are denoted by shorter lines drawn at an angle of 120° to each other.
The Newman’s projections for eclipsed and staggered conformations of ethane are as follows:
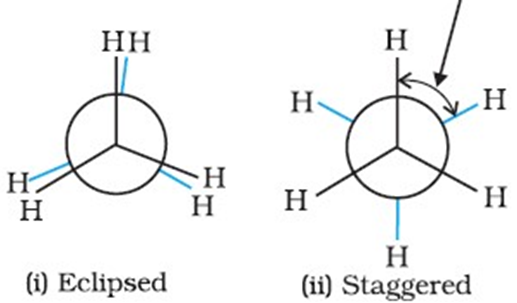
5. Aromatic hydrocarbon
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
AROMATIC HYDROCARBONS (ARENES)
- Most of the aromatic compounds have pleasant smell (aroma means pleasant smelling) and most of them contain benzene ring.
- Aromatic compounds containing benzene ring are called benzenoid compounds and those which do not contain benzene ring are called non-benzenoid compounds.
Structure of Benzene
- The molecular formula of benzene is C6H6, which indicates a high degree of unsaturation.
- But benzene was found to be a stable molecule and form a triozonide which indicates the presence of three double bonds.
- Also it produces only one monosubstituted derivative which indicates that all the six carbon and six hydrogen atoms of benzene are identical.
On the basis of these observations, August Kekulé proposed the following structure for benzene having cyclic arrangement of six carbon atoms with alternate single and double bonds.
The Kekule structure indicates the possibility of two isomeric1,2-disubstituted derivatives. But actually benzene forms only one 1,2-disubstituted derivative.
In order to overcome this problem, Kekule suggested the concept of oscillating nature of double bonds in benzene.
Kekule structure could not explain the stability of benzene and the preference of benzene to substitution reaction rather than addition reaction.
Resonance concept of Benzene
According to this concept, benzene is a hybrid of the following two resonace structures.
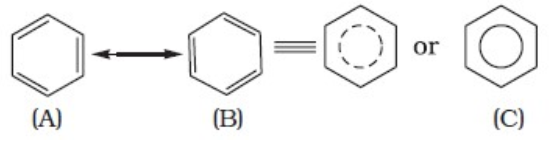
The actual structure of benzene is not A or B . it is in between thes two resonating structures. So benzene is denoted by a hexagon with a dotted circle, which represents the delocalised π-electrons.
Orbital Overlap Concept of Benzene
- In Benzene all the six carbon atoms are sp2 hybridized. Two sp2 hybrid orbitals of each carbon atom overlap with sp2 hybrid orbitals of adjacent carbon atoms to form six C—C sigma bonds which are in the hexagonal plane.
- The remaining one sp2 hybrid orbital of each carbon atom overlaps with 1s orbital of a hydrogen atom to form six C—H sigma bonds.
Now each carbon atom contains one unhybridised p orbital perpendicular to the plane of the ring. They overlap laterally to form three π-bonds. There are two possible overlapping.
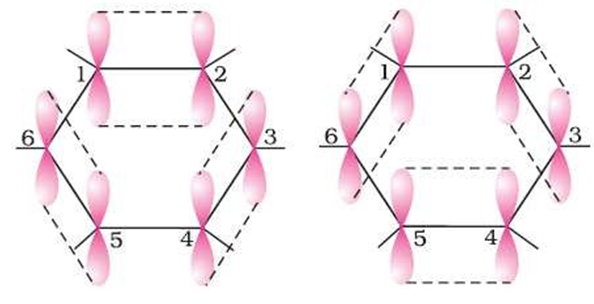
These give two Kekule structures with localized π electrons. But in benzene all the C-C bonds are identical and the bond length is 139 pm. To explain this, it is suggested that the p-orbitals of all the C atoms overlap each other. Thus in benzene, there is an electron cloud in the form two rings one above and one below the hexagonal ring as follows:
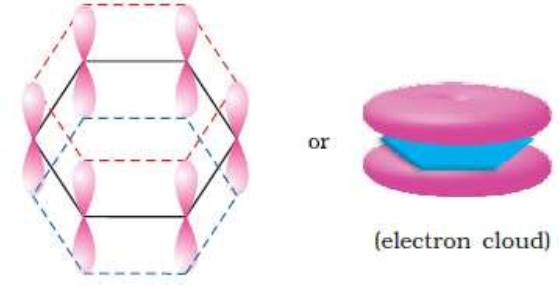
So the six π electrons are delocalised and can move freely about the six carbon nuclei. Presence of delocalised π electrons in benzene makes it more stable. The delocalised π electrons can be denoted by a circle inside a hexagonal ring. So benzene is best represented as:
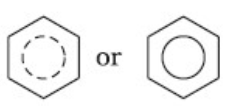
Aromaticity
- Aromaticity is defined by a rule called ‘Huckel rule’. According to this rule, “cyclic, planar, conjugated systems containing (4n+2) π electrons are aromatic”. Where n is the number of rings. n may be 1,2,3,….
- For benzene n = 1, so it should contain 6 delocalised π electrons. If n = 2, the no. of delocalised π electrons =10 and so on. Example for some aromatic compounds are:
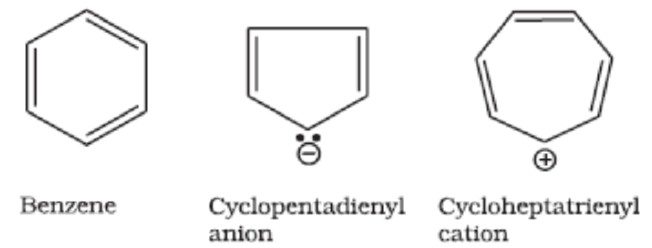
Preparation of Benzene
1. Cyclic polymerisation of ethyne (acetylene):
3 C2H2 Red hot iron tube & 873K C6H6
2.Decarboxylation of aromatic acids: Sodium salt of benzoic acid on heating with sodalime gives benzene.
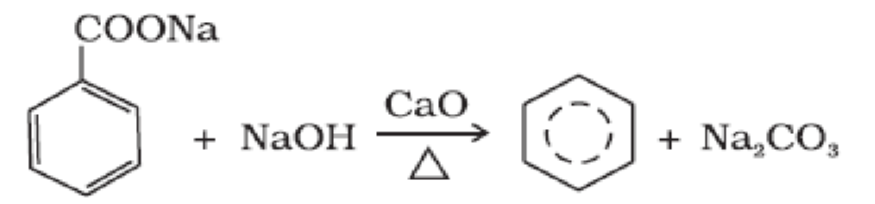
3.Reduction of phenol: Phenol is reduced to benzene by passing its vapours over heated zinc dust.
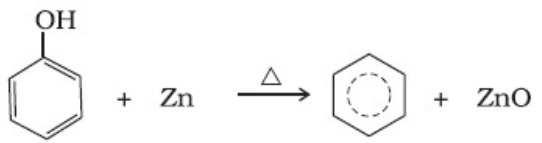
Chemical Properties
Aromatic compounds generally undergo electrophilic substitution reactions. Under special conditions, they can also undergo addition and oxidation reactions.
I) Electrophilic Substitution Reactions
These are reactions in which a weak electrophile is replaced by a strong electrophile. The important electrophilic substitution reactions are Nitration, Sulphonation, Halogenation and Friedel-Crafts alkylation and acylation.
1. Nitration: It is the introduction of nitro (-NO2) group to a benzene ring. For this benzene is heated with a mixture of conc. HNO3 and conc. H2SO4 (nitrating mixture).
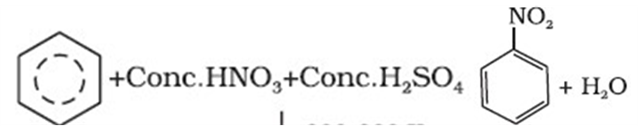
2. Halogenation: It is the introduction of halo (-X) group to a benzene ring. For this benzene is treated with a halogen (Cl2 or Br2) in presence of Lewis acids like anhydrous FeCl3, FeBr3 or AlCl3.
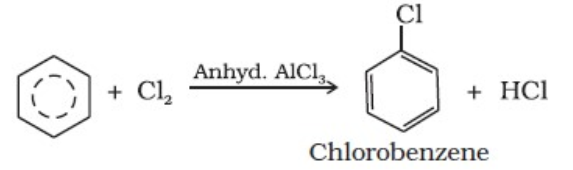
3. Sulphonation: It is the introduction of sulphonic acid (-SO3H) group to a benzene ring. It is carried out by heating benzene with fuming sulphuric acid (H2S2O7 or oleum).
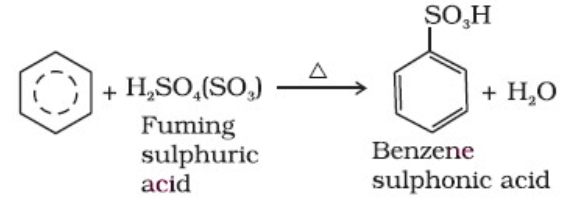
4. Friedel-Craft’s reaction: It is the introduction of alkyl (-R) group or acyl (-CO-R) group to a benzene ring. It is of two types:
a) Friedel-Craft’s Alkylation reaction: It is the introduction of alkyl (-R) group to a benzene ring. Here the reagents used are alkyl halide in presence of anhydrous AlCl3.
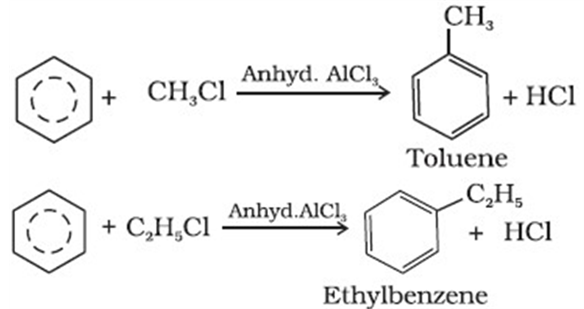
b) Friedel-Craft’s Acylation reaction: It is the introduction of acyl (-CO-R) group to a benzene ring. Here the reagents used are acyl halide in presence of anhydrous AlCl3.
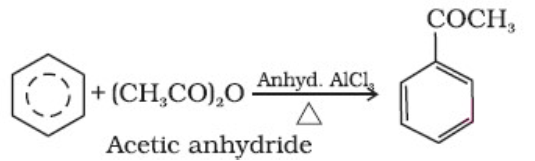
5. Benzene on treatment with excess of chlorine in the presence of anhydrous AlCl3 in dark to form hexachlorobenzene (C6Cl6).
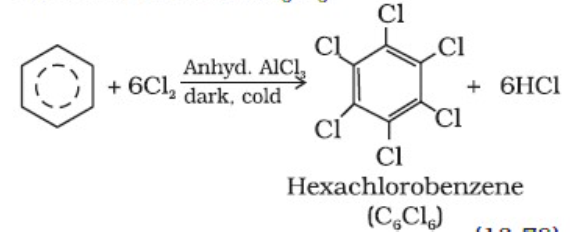
II) Addition Reactions
1. Addition of H2: Benzene add hydrogen in presence of nickel catalyst at high temperature and pressure to form cyclohexane.
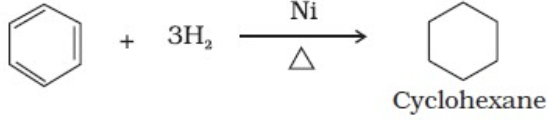
2. Addition of halogen: Benzene adds chlorine in presence of uv light to form benzene hexachloride (BHC). It is also known as Gammexane or Lindane or 666
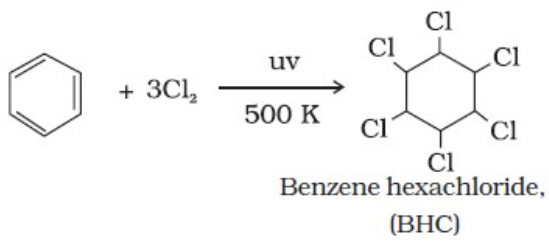
Directive influence of a functional group in mono-substituted benzene
- When monosubstituted benzene undergoes further substitution, two types of products are formed – either ortho and para products or meta product.
- This behaviour depends on the nature of the substituent already present in the benzene ring. This is known as directive influence of substituents.
There are two types of substituents – ortho and para directing groups and meta directing groups.
1. Ortho and para directing groups:
- The groups which direct the incoming group to ortho and para positions are called ortho and para directing groups.
- Example for such groups are –OH, –NH2, –NHR, -NHCOCH3, –OCH3, –CH3, –C2H5 etc.
- Generally, orho-para directing groups are activating groups, since they increases the electron density on benzene ring. Or, they activate the benzene ring for the attack by an electrophile.
Halogens are deactivating eventhough they are ortho-para directing. This is because of their strong –I effect.
2. Meta direcing groups:
- The groups which direct the incoming group to meta position are called meta directing groups.
- They are generally deactivating groups, since they reduces the electron density on benzene ring.
- Examples are –NO2, –CN, –CHO, –COR, –COOH, –COOR, –SO3H, etc
6. Carcinogenicity and toxicity
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
CARCINOGENICITY AND TOXICITY
Benzene and polynuclear hydrocarbons containing more than two benzene rings fused together are toxic and said to possess cancer producing (carcinogenic) property. Such polynuclear hydrocarbons are formed on incomplete combustion of organic materials like tobacco, coal and petroleum. They enter into human body and undergo various biochemical reactions and finally damage DNA and cause cancer. Some of the carcinogenic hydrocarbons are given below
2. Atmospheric pollution
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ATMOSPHERIC POLLUTION
• Troposphere
The lowest region of atmosphere, in which the human beings along with other organisms live, is called troposphere.
It extends to the height of about 10 km from the sea level. It contains air, water vapours, clouds etc. The pollution in this region is caused by some poisonous gases, smoke fumes, smog etc.
• Stratosphere
It extends from height of 10 to 50 km above the sea level. Ozone and some other gaseous substances present in this region are responsible for the pollution.
• Tropospheric Pollution
Pollution in this region is caused by the presence of undesirable gaseous particles like oxides of sulphur, nitrogen and carbon, hydrocarbons along with solid particles like dust, mist, fumes, smoke etc.
• Oxides of Sulphur
These are produced when coal containing sulphur is burnt.
S + O2 → SO2
SO2 + ½ O2 → SO3
SO3 + H2O → H2SO4
It is also produced during volcanic eruptions.
Harmful effects:
(i) It is poisonous to both animals and plants.
(ii) A very high concentration of S02 may cause respiratory diseases e.g., asthma,bronchitis, emphysema in human beings.
(iii) It causes irritation to the eyes, resulting in tears and redness.
(iv) Its high concentration leads to the stiffness of flower buds.
(v) Particulate matter present in the air can catalyse the formation of sulphur trioxide from sulphur dioxide.
2SO2(g) + O2(g) → 2SO3
O3 and H2O2 also promote this reaction.
SO2(g) + O3(g) → SO3(g) + O2(g)
SO2(g) + H2O2(l) → H2SO4(aq)
• Oxides of Nitrogen
Main oxides of nitrogen are nitric oxide (NO) and nitrogen dioxide (NO2).
Major Sources:
(i) Lightning discharge results in the combination of N2 and 02 to form NO.
(ii) Combustion of gasoline in automobilies, burning of hydrocarbons and coal etc.
Harmful effects:
Nitric oxide itself is not harmful to human beings, but it is very unstable and changes to nitrogen dioxide which is toxic in nature. These effects are as follows:
(i) It reacts with Ozone (03) present in the atmosphere and thus decrease the density of Ozone.
(ii) It affects the respiratory system and damages the lungs.
(iii) Higher concentrations of N02 damage the leaves of plants and retard the rate of photosynthesis.
(iv) It causes cracks in rubber.
(v) Nitrogen dioxide is also harmful to various textile fibres and metals.
• Hydrocarbons
Incomplete combustion of fossil fuel in industry and thermal power plants and the exhaust of automobiles release hydrocarbons into the atmosphere constantly causing pollution. Harmful Effects:
(i) They cause cancer.
(ii) Methane is one of the greenhouse gases.
(iii) They harm plants in various ways like breakdown of tissues, shedding of leaves etc.
• Oxides of Carbon
Carbon dioxide:
0.03% C02 is present in air by Volume.
Major Sources:
(i) By burning of fossil fuels.
(ii) By the decomposition of limestone during the manufacture of cement.
(iii) Emitted during volcanic eruptions.
(iv) C02 is released into the atmosphere by respiration.
Harmful effects:
Deforestation and burning of fossil fuel increases the C02 level which is mainly responsible for global warming.
Carbon Monoxide: Carbon Monoxide is a colourless and odourless gas.
Major Sources:
(i) Released by the automobile exhaust.
(ii) Incomplete combustion of coal, fire wood, petrol etc.
(iii) By the dissociation of C02 at high temperature.
Harmful effects:
(i) It binds to haemoglobin to form carboxyhaemoglobin which is more stable than oxygen-haemoglobin complex. Its concentration in blood when reaches to 3-4%, the oxygen carrying capacity of blood is greatly reduced.
The oxygen deficiency, results into headache, weak eyesight, nervousness etc.
(ii) It has harmful effects on plants when its concentration is (100 ppm or more).
• Global Wanning and Greenhouse Effect
Greenhouse Effect:
Some gases like carbondioxide, methane, ozone, water vapours, CFCs have the capacity to trap some of the heat radiations that are released from the earth or from sun. These gases are known as greenhouse gases and the effect is called greenhouse effect. This leads to global warming
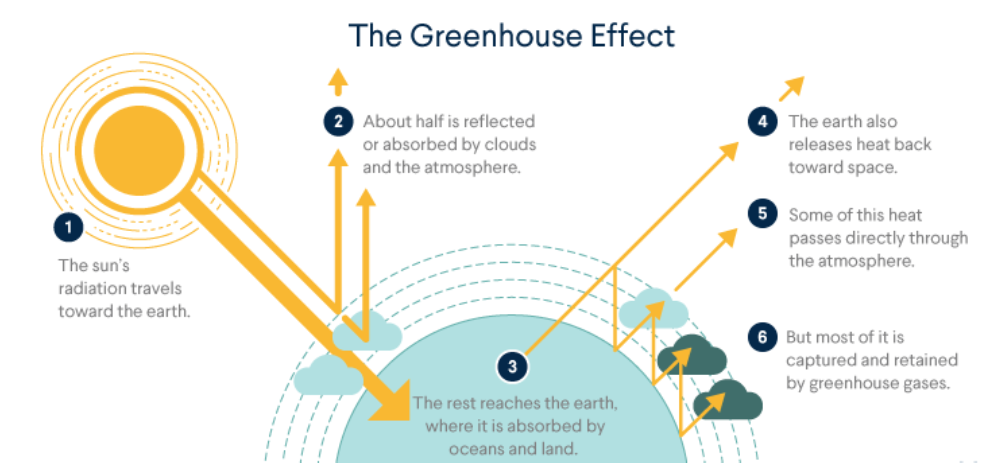
Consequences of global warming:
(i) It leads to melting of polar ice caps and flooding of low lying areas all over the earth.
(ii) Global rise in temperature increases the incidence of infectious diseases like dengue, malaria, yellow fever, sleeping sickness etc.
• Acid Rain
When the pH of the rain water drops below 5.6, it is known as acid rain.
Normal rain is slightly acidic due to dissolution of atmospheric carbon dioxide in water.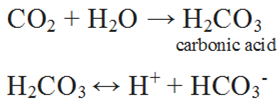
Oxides of nitrogen and sulphur released as a result of combustion of fossil fuels dissolve in water to form nitric acid and sulphuric acid.
4NO2 + O2 + 2H2O → 4HNO3
SO2 + ½ O2 → H2SO4
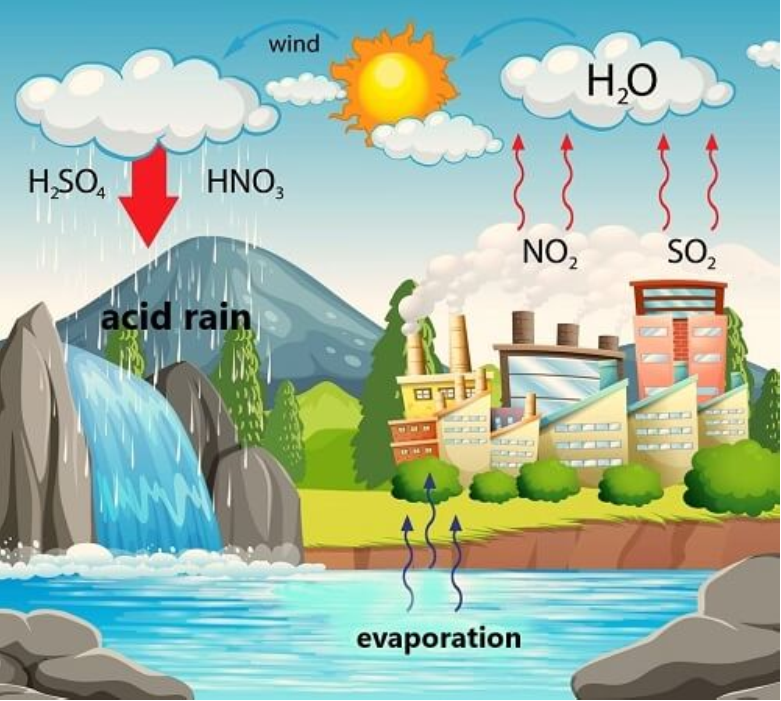
Harmful Effects of Acid Rain:
(i) It has harmful effects on trees and plants as it dissolves and washes away nutrients needed for their growth.
(ii) It has very bad effect on aquatic ecosystem.
(iii) Acid rain damages buildings and other structures made of stone or metal. Taj Mahal in India has beenaffected by acid rain.
CaCO3 (marble) + H2SO4 → CaSO3 + H2O + CO2
• Particulate Pollutants
Viable Particulates: They are minute living organisms that are dispersed in the atmosphere. e.g., bacteria, fungi) moulds, algae etc.
Non Viable Particulates:
(i) Smoke: It is the mixture of solid and liquid particles formed during combustion of organic matter,
Example: Cigarette smoke, smoke from burning of fossil fuel.
(ii) Dust: Composed of fine solid particles (over 2gm in diameter).
It is produced during crushing, grinding and attribution of solid particles.
(iii) Mist: These are produced due to the spray of liquids like herbicides and pesticides over the plants. They travel through air and form mist.
(iv) Fumes: They are generally released to the atmosphere by the metallurgical operations and also by several chemical reactions.
Harmful Effects of Particulate Pollutants:
(i) Fine particles less than 5 microns penetrate into the lungs. Inhalation of such particles can lead to serious lung diseases including lung cancer.
(ii) Suspended particles of bigger size can hinder the sun rays from reaching the earth surface. This can lower the temperature of earth and make the weather foggy.
• Smog
This is the common form of air pollution which is combination of smoke and fog.
Smog exists in two types:
(i) Classical Smog: Occurs in cool humid climate. It contains smoke, fog and sulphur dioxide. It is also called as reducing smog.
(ii) Photochemical Smog: This type of smog result from the action of sunlight on unsaturated hydrocarbons and nitrogen oxides released by the vehicles and industries. It has high concentration of oxidising agents and is therefore, called as oxidising smog.
Formation of Photochemical Smog
![]()
Oxygen atoms combine with air (O2) and form O3 .
O(g) + O2(g) ↔ O3(g)
NO(g) + O3(g) → NO2(g) + O2(g)
![]()
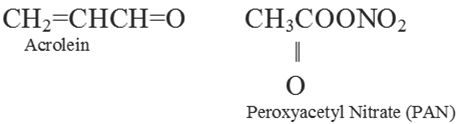
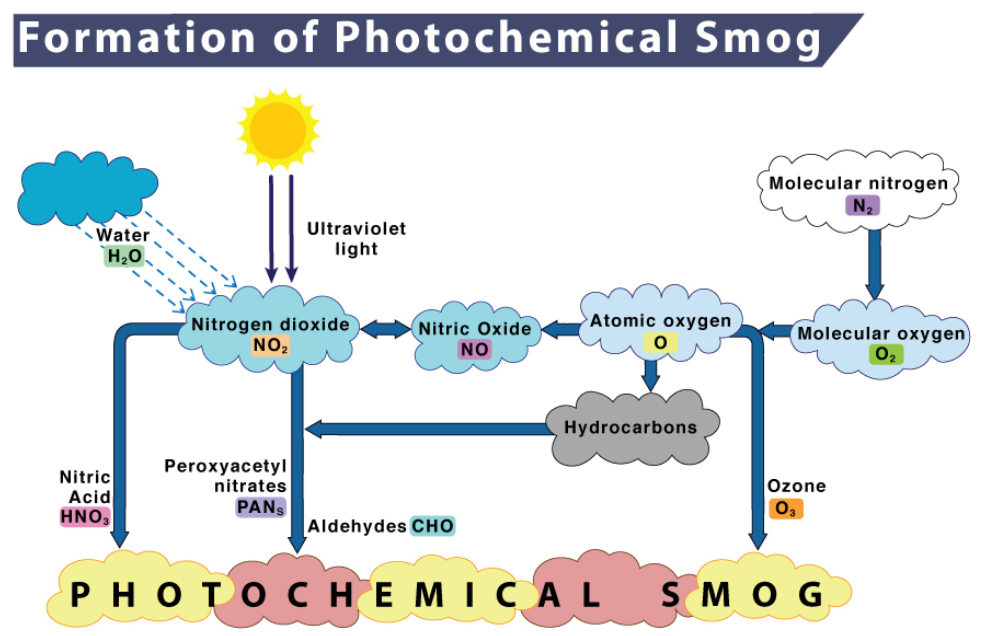
Harmful effects of photochemical smog:
(i) It can cause cough, bronchitis, irritation of respiratory system etc.
– To control this type of pollution the engines of the automobiles are fitted with catalytic converters to check the release of both oxides of nitrogen and hydrocarbons in the atmosphere.
– Some plants like Vitis, Pinus, Juniparus, Quercus, Pyrus can metabolise nitrogen
oxide and therefore, their plantation can be done.
• Stratospheric Pollution
Formation of Ozone: Ozone in the stratosphere is produced by UV radiations. When UV – radiations act on dioxygen (02) molecules, Ozone is produced.
![]()
![]()
Ozone is thermodynamically unstable and decomposes to molecular oxygen. Thus there exists an equilibrium between production and decomposition of Ozone molecules.
Depletion of Ozone layer:
Ozone blanket in the upper atmosphere prevent the harmful UV radiations from reaching earth.
But in recent years, there have been reports of depletion of this layer due to presence of ,certain chemicals in the stratosphere. Chlorofluorocarbons (CFCs), nitrogen oxides, chloride, CCl4 etc. are the chemicals responsible for depletion.
Cl2CF2 → •Cl + •CClF2
•Cl + O3 → ClO• + O2
ClO• + O• → •Cl + O2
Chlorofluorocarbons dissociate in the presence of light gives chlorine free radicals which catalyse the conversion of ozone into oxygen.
Effects of the depletion of Ozone layer:
(i) This leads to many diseases like skin cancer, sunburn, ageing of skin, cataract etc.
(ii) UV radiations can kill many phytoplanktons, damage the fish productivity.
(iii) It can decrease moisture content of the soil by increasing the evaporation of surface water.
(iv) UV radiations can damage paints and fibres, causing them to fade faster.
3. Water pollution
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
WATER POLLUTION
Presence of undesirable materials in water which is harmful for the human beings and plants is known as water pollution. Normal properties of the water can be changed by the presence of these foreign materials.
Maximum prescribed concentration of some metals in drinking water
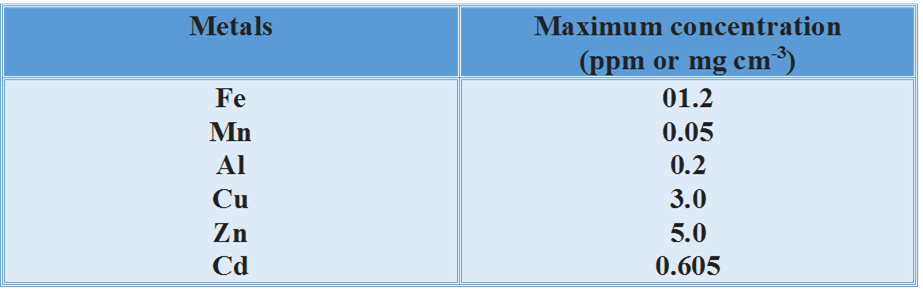
Major water pollutants
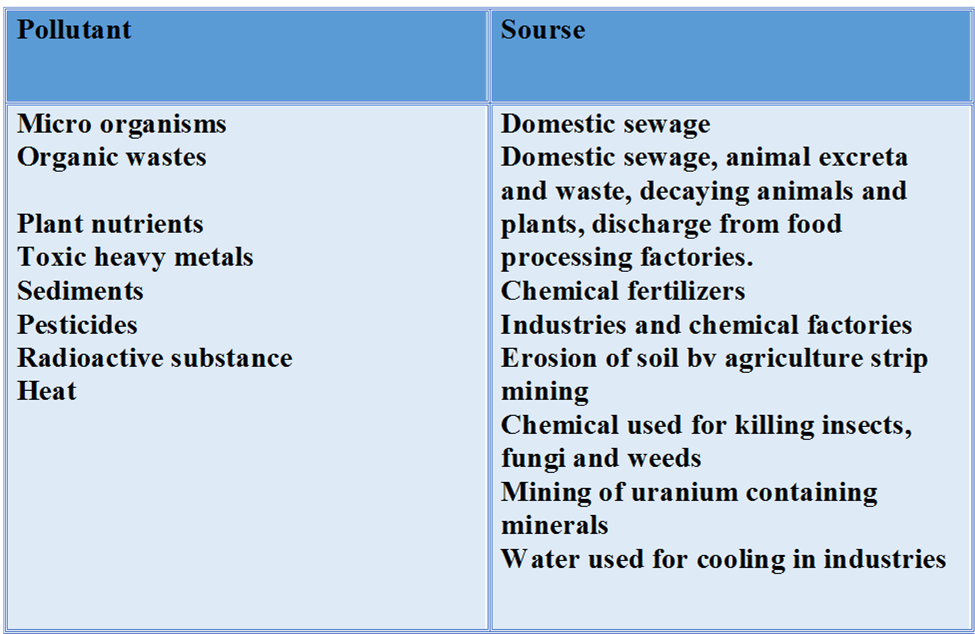
Causes of Water Pollution:
(i) Pathogens: Pathogens are the bacteria and the other organisms that enter water from domestic sewage and animal excreta.
Human excreta contain bacteria such as Escherichia coli and Streptococcus faecalis. It causes gastrointestinal diseases.
(ii) Organic Wastes: Organic matter such as leaves, grass, trash etc. can pollute water.
– Excessive growth of phytoplankton within water also pollute water.
– Large numbers of bacteria in water can consume oxygen dissolved in water by decomposing organic matter present in water.
– If the concentration of dissolved oxygen in water is below 6 ppm, the growth of fish gets inhibited.
– If too much of organic matter is added to water, all the available oxygen is used up. This can cause the death of the aquatic life.
• BOD (Biochemical Oxygen Demand)
It is defined as the amount of oxygen required by bacteria for the breakdown of the organic matter present in a certain volume of a sample of water.
The amount of BOD in water is a measure of the amount of organic material in the water. Clean water has BOD value of less than 5 ppm.
Highly polluted water could have a BOD value of 17 ppm or more.
• Chemical Pollutants
(i) Industrial Wastes: Chemical reactions carried in the industrial units also pollute water to a great extent. For example, lead, mercury, nickel, cobalt etc. These chemicals give very bad effect to the groundwater and waterbodies are polluted due’ to the chemical reactions known as leaching.
Organic chemicals like petroleum products also pollute many sources of water e.g., major oil spills in oceans.
(ii) Pesticides: These are mostly chlorinated hydrocarbons, organophosphates and metallic salts etc. They dissolve in water to small extent and pollute it. Since all the pesticides are toxic in nature, they are injurious to both plants and animals.
(iii) Polychlorinated biphenyls (PCBS): These are the chemical compounds used as fluids in transformers and capacitors. These are released in atmosphere as vapours. They mix with rain water and thus contaminate the water.
(iv) Eutrophication: The process in which algae like organisms reduce dissolved oxygen in water is called as eutrophication. It is harmful for aquatic life.
• International Standards for Drinking Water
Fluoride: Concentration of fluoride up to 1 ppm or 1 mg dm-3, is not harmful for human
beings if it is used as drinking water. The F~ ions make the enamel on teeth much harder by converting hydroxyapatite [3Ca3(P04)2- Ca(OH)2] the enamel on the surface of the teeth, into much harder fluorapatite, [3Ca3(P04)2- CaF2]. Concentration of F~ above 2 ppm causes brown mottling of teeth. Excess of fluoride is harmful to bones also.
Lead: Upper limit concentration of lead in drinking water is about 50 ppm. Lead can damage kidney, lever, reproductive system etc.
Sulphate: At moderate level it is harmless but excess is harmful.
Nitrate: The maximum limit of nitrate should be 50 ppm. Excess nitrate in drinking water can cause diseases such as methemoglobinemia (blue baby syndrome).
Chemical Oxygen Demand (COD): Water is treated with K2Cr207 in acidic medium to oxidise polluting substance which cannot be oxidised by microbial oxidation. The remaining is determined by back titration with suitable reducing agent.
From the concentration of K2Cr207 consumed, the amount of O2 used in the oxidation is calculated.
K2Cr2O7(aq) + 4H2SO4(aq) → K2SO4(aq) + Cr2(SO4)3(aq) + 3H2O + 3O
4. Soil pollution
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
SOIL POLLUTION—SOURCES OF SOIL POLLUTION
Pesticides: It can be classified as:
(i) Insecticide: The most common insecticides are chlorinated hydrocarbons like DDT, BHC etc.
As they are not much soluble in water, they stay in the soil for long time. They are ‘ absorbed by the soil and contaminate root crops like radish, carrot etc.
(ii) Herbicides: These are the compounds used to control weeds, namely, sodium chlorate (NaCl03) and sodium arsenite (Na3As03) are commonly used herbicides but arsenic compounds, being toxic are no longer preferred.
Fungicides: Organo-mercury compounds are the most common fungicides. Its dissociation in soil produces mercury which is highly toxic and harmful for the crops. i Industrial Waste: It has seen that most of the industrial wastes are thrown into water or dumped into the soil. These industrial wastes contain huge amounts of toxic chemicals which are mostly non-bidegradable. For example, metal processing industries, mining cement, glass industries, petroleum industry etc., fertilizer industry produce gypsum.
The disposal of non-biodegradable industrial solid waste is not done by suitable methods i and cause many serious problems.
INDUSTRIAL WASTE
The waste materials generated by industries or industrial processes, is called industrial waste. It includes chemicals, trash, oils, solvents, dirt and gravel, many harmful gases etc. These are dumped in seas, rivers or land without adequate treatment. Thus, become a large source of environmental pollution.
Types of industrial wastes
Industrial waste can be divided into following two types
- Biodegradable industrial waste
- Non – biodegradable industrial waste
Biodegradable wastes – Those waste materials which can be decomposed into simpler unharmful substances by the action of microorganisms are called biodegradable wastes. Some industries such as the paper industry, food industry, sugar industry, wool industry etc. mostly produce biodegradable industrial wastes. Management of these wastes can be done at low cost and easily.
Non-biodegradable wastes – Non-biodegradable waste cannot be further decomposed via the action of the microorganisms. Such waste is the major source of toxins in the landfills. Chemicals, metals, plastics, paints, rubber etc. are examples of non-biodegradable wastes. These materials can remain as landfills for thousands of years without any damage. Toxins from metals and plastics get soaked into the earth and pollute the soil and water sources. Cleaning materials such detergent, phenols etc. producing industries, coal industries, dying industries etc. produce a large amount of non-biodegradable industrial waste. These types of wastes are difficult to manage and very toxic in nature.
Effects of Industrial Waste
Industrial waste is very harmful for us and our environment. Few impacts are stated below –
- Liquid industrial waste which is thrown into the sea is at an alarmingly dangerous level for marine ecosystems.
- Industries release many harmful gases such as carbon dioxide, sulfur dioxide, nitrogen oxides etc. which cause air pollution.
- In industrial wastewater nitrates and phosphates are there which often cause eutrophication.
- Generally, air around industries is highly polluted and causes skin, eyes, throat, nose and lungs diseases.
- Industries use large quantities of water and also release a huge quantity of wastewater which contain many harmful chemicals and heavy metals. This wastewater pollutes natural sources of water and ultimately our health and environment.
- It is one of the main causes of global warming.
- Industrial wastewater destroys useful bacteria and other microorganisms present in soil.
- Some industries cause sound pollution as well.
- Industrial wastes and industries are destroying natural habitat of many species and responsible for wildlife extinction.
Proper disposal and treatment is the only solution of prevention from effects of industrial wastes.
Management of Industrial Waste
Management of industrial solid waste is not the responsibility of local bodies or governments. Industries which are generating these solid wastes should manage such wastes by themselves. They need to take authorization from the pollution control board as well. Different procedures and methods are used to manage industrial waste. Although some basic steps involved in all processes are the same. Those basic steps are as follows –
- Analysis or Segregation
- Collection
- Transportation
- Recovery
- Recycling
- Disposal
Analysis or Segregation – Industrial waste is segregated or analyzed, and some biodegradable wastes or recyclable material are kept separately. Industries should segregate waste materials in different categories such as biodegradable, non-biodegradable, hazardous waste etc.
Collection and Transportation – Industrial waste must be collected and transported to waste management plants.
Recovery – In waste management plants recovery should be done. It means useful materials should be recovered from industrial wastes during treatment in waste management plants.
Recycling and Disposal – If during recovery we get any useful materials then recycling should be done and disposal should be done of waste and harmful materials.
STRATEGIES TO CONTROL ENVIRONMENTAL POLLUTION
Environmental pollution is also a major global concern due to the harmful effects of pollution on a person’s health and on the environment as well. Some of the strategies that are to be followed to lessen environmental pollution include the following:
- Stop smoking or at least follow “No Smoking” regulations at public places.
- Do not use open fires for waste disposal.
- Instead of plastic, use eco-friendly or biodegradable products, because plastic-like products are highly toxic in nature.
- Maintain proper waste disposal, especially for toxic wastes, and plan some strategies to reduce waste.
- Do not litter in public places, and some anti-litter campaigns should be run to educate the public.
GREEN CHEMISTRY
Green Chemistry is a way of thinking and is about utilising the knowledge and principles of
chemistry that would control the increasing environmental pollution.
Green chemistry in day-to-day life:
(i) Dry-Cleaning of clothes and laundary: Replacement of halogenated solvent like (CCl4) by liquid C02 which is less harmful to groundwater. Hydrogen peroxide (H202) is used for the purpose of bleaching clothes.
(ii) Bleaching of Paper: In place of chlorine H202 is used for the bleaching of paper,
(iii) Synthesis of Chemicals: Ethahal (CH3CHO) is prepared by step oxidation of ethene. Such as,
![]()
Definition of green chemistry
Green chemistry is the design of chemical products and processes that reduce or eliminate the use or generation of hazardous substances. Green chemistry applies across the life cycle of a chemical product, including its design, manufacture, use, and ultimate disposal.
Green chemistry:
- Prevents pollution at the molecular level
- Is a philosophy that applies to all areas of chemistry, not a single discipline of chemistry
- Applies innovative scientific solutions to real-world environmental problems
- Results in source reduction because it prevents the generation of pollution
- Reduces the negative impacts of chemical products and processes on human health and the environment
- Lessens and sometimes eliminates hazard from existing products and processes
- Designs chemical products and processes to reduce their intrinsic hazards
How green chemistry differs from cleaning up pollution
Green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products.
This is unlike cleaning up pollution (also called remediation), which involves treating waste streams (end-of-the-pipe treatment) or cleanup of environmental spills and other releases. Remediation may include separating hazardous chemicals from other materials, then treating them so they are no longer hazardous or concentrating them for safe disposal. Most remediation activities do not involve green chemistry. Remediation removes hazardous materials from the environment; on the other hand, green chemistry keeps the hazardous materials out of the environment in the first place.
If a technology reduces or eliminates the hazardous chemicals used to clean up environmental contaminants, this technology would qualify as a green chemistry technology. One example is replacing a hazardous sorbent [chemical] used to capture mercury from the air for safe disposal with an effective, but non-hazardous sorbent. Using the non-hazardous sorbent means that the hazardous sorbent is never manufactured and so the remediation technology meets the definition of green chemistry.
Green chemistry's 12 principles
These principles demonstrate the breadth of the concept of green chemistry:
1. Prevent waste: Design chemical syntheses to prevent waste. Leave no waste to treat or clean up.
2. Maximize atom economy: Design syntheses so that the final product contains the maximum proportion of the starting materials. Waste few or no atoms.
3. Design less hazardous chemical syntheses: Design syntheses to use and generate substances with little or no toxicity to either humans or the environment.
4. Design safer chemicals and products: Design chemical products that are fully effective yet have little or no toxicity.
5. Use safer solvents and reaction conditions: Avoid using solvents, separation agents, or other auxiliary chemicals. If you must use these chemicals, use safer ones.
6. Increase energy efficiency: Run chemical reactions at room temperature and pressure whenever possible.
7. Use renewable feedstocks: Use starting materials (also known as feedstocks) that are renewable rather than depletable. The source of renewable feedstocks is often agricultural products or the wastes of other processes; the source of depletable feedstocks is often fossil fuels (petroleum, natural gas, or coal) or mining operations.
8. Avoid chemical derivatives: Avoid using blocking or protecting groups or any temporary modifications if possible. Derivatives use additional reagents and generate waste.
9. Use catalysts, not stoichiometric reagents: Minimize waste by using catalytic reactions. Catalysts are effective in small amounts and can carry out a single reaction many times. They are preferable to stoichiometric reagents, which are used in excess and carry out a reaction only once.
10. Design chemicals and products to degrade after use: Design chemical products to break down to innocuous substances after use so that they do not accumulate in the environment.
11. Analyse in real time to prevent pollution: Include in-process, real-time monitoring and control during syntheses to minimize or eliminate the formation of byproducts.
12. Minimize the potential for accidents: Design chemicals and their physical forms (solid, liquid, or gas) to minimize the potential for chemical accidents including explosions, fires, and releases to the environment.
Green chemistry's roots in the Pollution Prevention Act of 1990
To stop creating pollution in the first place became America's official policy in 1990 with the Federal Pollution Prevention Act .
The law defines source reduction as any practice that:
- Reduces the amount of any hazardous substance, pollutant, or contaminant entering any waste stream or otherwise released into the environment (including fugitive emissions) prior to recycling, treatment, or disposal.
- Reduces the hazards to public health and the environment associated with the release of such substances, pollutants, or contaminants.
The term "source reduction" includes:
- Modifications to equipment or technology
- Modifications to process or procedures
- Modifications, reformulation or redesign of products
- Substitution of raw materials
- Improvements in housekeeping, maintenance, training, or inventory control
Section 2 of the Pollution Prevention Act establishes a pollution prevention hierarchy, saying:
- The Congress hereby declares it to be the national policy of the United States that pollution should be prevented or reduced at the source whenever feasible;
- Pollution that cannot be prevented should be recycled in an environmentally safe manner, whenever feasible;
- Pollution that cannot be prevented or recycled should be treated in an environmentally safe manner whenever feasible; an
- Disposal or other release into the environment should be employed only as a last resort and should be conducted in an environmentally safe manner.
Green chemistry aims to design and produce cost-competitive chemical products and processes that attain the highest level of the pollution-prevention hierarchy by reducing pollution at its source.
For those who are creating and using green chemistry, the hierarchy looks like this:
- Source Reduction and Prevention of Chemical Hazards
- Designing chemical products to be less hazardous to human health and the environment*
- Making chemical products from feedstocks, reagents, and solvents that are less hazardous to human health and the environment*
- Designing syntheses and other processes with reduced or even no chemical waste
- Designing syntheses and other processes that use less energy or less water
- Using feedstocks derived from annually renewable resources or from abundant waste
- Designing chemical products for reuse or recycling
- Reusing or recycling chemicals
- Treating chemicals to render them less hazardous before disposal
- Disposing of untreated chemicals safely and only if other options are not feasible
*Chemicals that are less hazardous to human health and the environment are:
- Less toxic to organisms
- Less damaging to ecosystems
- Not persistent or bio accumulative in organisms or the environment
- Inherently safer to handle and use because they are not flammable or explosive
• Environmental pollution: It is the effect of undesirable changes in the surroundings that have harmful effects on plants, animals, and human beings.
• Troposphere: The lowest region of atmosphere which extends up to the height of 10 km from sea level in which man and other living organism exists.
• Stratosphere: It is above troposhere between 10 to 50 km above the sea level.
• Acid rain: It is caused by the presence of oxides of sulphur and nitrogen and C02 in the atmosphere. The pH of the rain drops below 5.6, and it becomes acidic.
• Greenhouse gases: Some gases like carbon dioxide, methane, ozone, water vapours, CFCs have the capacity to trap some of the heat radiations from the earth or from the sun. This leads to global warming.
• Eutrophication: When phosphate ion increases in water it increases the growth of algae which consume the dissolved oxygen in water consequently aquatic life is adversely affected. This results in loss of biodiversity and the phenomenon is known as Eutrophication.
• COD (Chemical Oxygen Demand): It is calculated as the amount of oxygen required to oxidise the polluting substances. It is measured by treating the given sample of water with an oxidising agent, generally K2Cr207in the presence of dil. H2S04.
3. Alkenes
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ALKENES
- Alkenes are unsaturated hydrocarbons containing at least one double bond.
- They are also known as olefins (oil forming) since the first member, ethylene or ethene (C2H4) was found to form an oily liquid on reaction with chlorine.
- The general formula for alkenes is CnH2n. Isomerism in alkenes
- Alkenes show structural and stereo isomerism.
- The important structural isomerisms shown by alkenes are chain isomerism and position isomerism.
- The stereo isomerism shown by alkenes is geometrical isomerism.
Geometrical Isomerism
- The isomerism arising due to the difference in the spatial arrangement of atoms around carbon-carbon double bond is called geometrical isomerism.
- Such isomers are called geometrical isomers. It is a type of stereo isomerism.
- Geometrical isomerism arising due to the restricted rotation about carbon-carbon double bond.
- There are two types of geometrical isomers – cis isomer and trans isomer.
- Isomer in which identical atoms or groups are on the same side of the double bond is called cis isomer.
- If the identical groups or atoms are on the opposite side of the double bond, it is called trans isomer.
Compounds with formula YX C = C XY can show geometrical isomerism as follows:

e.g. But-2-ene

Due to different arrangement of atoms or groups in space, these isomers differ in their physical properties like melting point, boiling point, dipole moment, solubility etc
Cis form of alkene is found to be more polar than the trans form. In the case of solids, the trans isomer has higher melting point than the cis form.
PREPARATION OF ALKENES
1. From alkynes:
Alkynes on partial reduction with dihydrogen in the presence of palladised charcoal partially deactivated with sulphur compounds or quinoline give alkenes.
- Partially deactivated palladised charcoal is known as Lindlar’s catalyst. Alkenes thus obtained are having cis geometry.

If we use sodium in liquid ammonia as the reducing agent, we get trans alkene

2. From Alkyl halides:
- Alkyl halides (R-X) on heating with alcoholic potash, eliminate one molecule of hydrogen halide to form alkenes.
- This reaction is known as dehydrohalogenation i.e., removal of hydrogen halide.
- Since hydrogen atom is eliminated from the β carbon atom, the reaction is also known as β-elimination reaction.
CH3-CH2X → CH2 = CH2 + HX
CH3-CH2-CH2Br → CH3-CH = CH2 + HBr
3. From vicinal dihalides:
- Dihalides in which two halogen atoms are attached to two adjacent carbon atoms are known as vicinal (vic) dihalides.
- Vicinal dihalides on treatment with zinc metal lose a molecule of ZnX2 to form an alkene.
- This reaction is known as dehalogenation (i.e. elimination of halogen molecule).
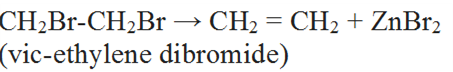
CH3-CHBr-CH2Br + Zn → CH3-CH = CH2 + ZnBr2
4. From alcohols:
- Alcohols when heated with concentrated sulphuric acid undergo dehydration (elimination of water molecule) to form alkenes.
- This reaction is also the example of β-elimination reaction since –OH group is eliminated from the β-carbon atom.
CH3-CH2-OH → CH2 = CH2 + H2O
Chemical Properties
1. Addition of hydrogen:
Alkenes add hydrogen in presence of finely divided Ni, Pd or Pt to form alkanes.
CH2 = CH2 + H2 → CH3-CH3
2. Addition of halogen:
Alkenes add halogen (Cl2 orBr2) to form vicinal dihalides.
CH2 = CH2 + X2 → CH2X-CH2X
When Br2 dissolved in CCl4 (carbon tetrachloride) is added to unsaturated compounds (alkenes or alkynes), the reddish orange colour of bromine solution is discharged. This reaction is used as a test for unsaturation.
CH2 = CH2 + Br2 → CH2Br-CH2Br
3. Addition of hydrogen halide:
Alkenes add hydrogen halide to form alkyl halides.
CH2 = CH2 + HX → CH3-CH2X
CH2 = CH2 + HBr → CH3-CH2Br
![]()
Addition of HBr to unsymmetrical alkenes (Markovnokov’s Rule)
When HBr is added to propene, we get 2 products – 1-bromopropane and 2-bromopropane.
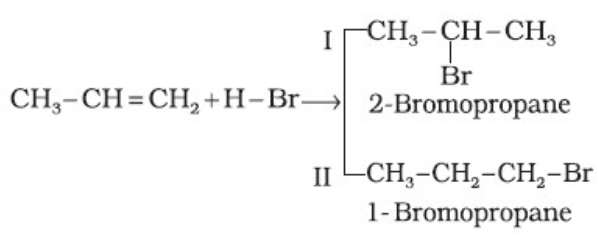
Here the major product is determined by a rule called Markovnikov rule (Markownikoff’s rule). The rule states that “when an unsymmetrical reagent is added to an unsymmetrical alkene, the negative part of the addendum (adding molecule) gets attached to the carbon containing lesser number of hydrogen atoms” (less hydrogenated C atom).
Thus in the above reaction 2-bromopropane is the major product.
In the presence of peroxide, addition of HBr to unsymmetrical alkenes takes place against Markovnikov rule. This is known as peroxide or Kharash effect or anti-Markovnikov addition reaction.![]()
4. Addition of water:
Alkenes add water in presence of a few drops of concentrated sulphuric acid to form alcohols. The reaction occurs according to the Markovnikov rule.![]()
5. Oxidation:
(i) When oxidised using cold and dilute aqueous solution of potassium permanganate (KMnO4) [commonly called Baeyer’s reagent], alkenes give vicinal glycols.
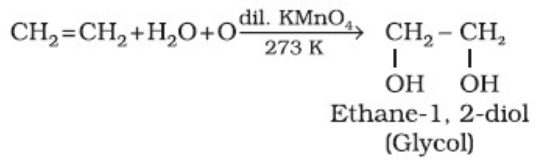
When KMnO4 is added to alkene, its pink colour gets discharged. So this reaction is also used as a test for unsaturation.
(ii) Acidic KMnO4 or acidic potassium dichromate (K2Cr2O7) oxidises alkenes to ketone or acids depending on the nature of the alkene.
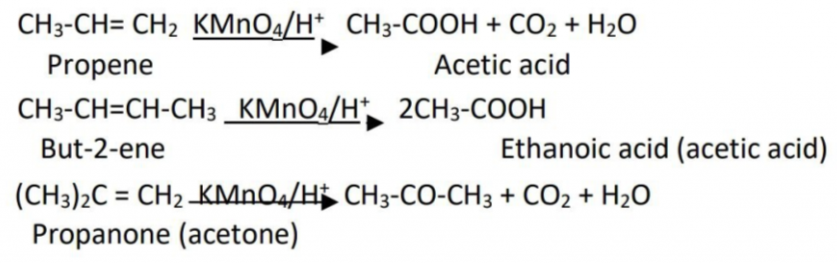
6. Ozonolysis: Alkenes add ozone to form an ozonide which on hydrolysis in presence of Zn to form carbonyl compounds (Aldehydes or ketones).
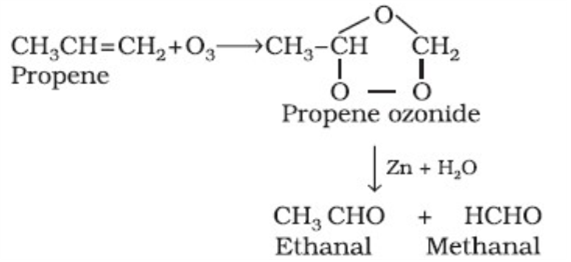
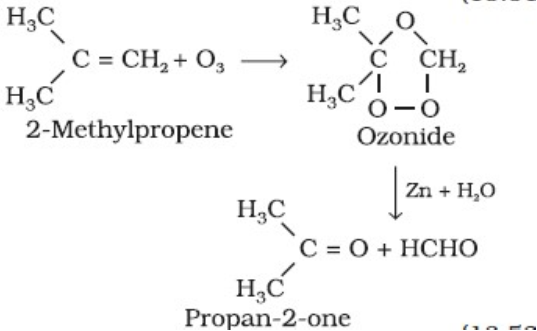
7. Polymerisation:
- The complex organic molecules formed by the combination of simple molecules are called polymers (macromolecules) and the reaction is called polymerisation reaction.
- The simple molecule from which a polymer is formed is called monomer.
3. Alkenes
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ALKENES
- Alkenes are unsaturated hydrocarbons containing at least one double bond.
- They are also known as olefins (oil forming) since the first member, ethylene or ethene (C2H4) was found to form an oily liquid on reaction with chlorine.
- The general formula for alkenes is CnH2n. Isomerism in alkenes
- Alkenes show structural and stereo isomerism.
- The important structural isomerisms shown by alkenes are chain isomerism and position isomerism.
- The stereo isomerism shown by alkenes is geometrical isomerism.
Geometrical Isomerism
- The isomerism arising due to the difference in the spatial arrangement of atoms around carbon-carbon double bond is called geometrical isomerism.
- Such isomers are called geometrical isomers. It is a type of stereo isomerism.
- Geometrical isomerism arising due to the restricted rotation about carbon-carbon double bond.
- There are two types of geometrical isomers – cis isomer and trans isomer.
- Isomer in which identical atoms or groups are on the same side of the double bond is called cis isomer.
- If the identical groups or atoms are on the opposite side of the double bond, it is called trans isomer.
Compounds with formula YX C = C XY can show geometrical isomerism as follows:
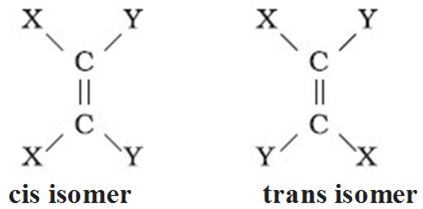
e.g. But-2-ene
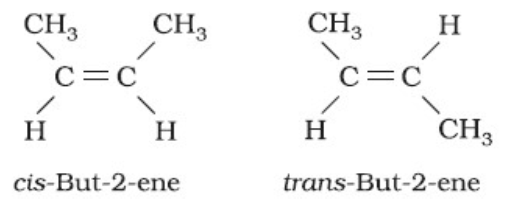
Due to different arrangement of atoms or groups in space, these isomers differ in their physical properties like melting point, boiling point, dipole moment, solubility etc
Cis form of alkene is found to be more polar than the trans form. In the case of solids, the trans isomer has higher melting point than the cis form.
PREPARATION OF ALKENES
1. From alkynes:
Alkynes on partial reduction with dihydrogen in the presence of palladised charcoal partially deactivated with sulphur compounds or quinoline give alkenes.
- Partially deactivated palladised charcoal is known as Lindlar’s catalyst. Alkenes thus obtained are having cis geometry.
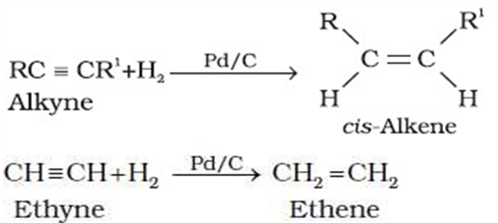
If we use sodium in liquid ammonia as the reducing agent, we get trans alkene
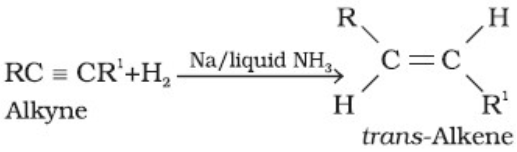
2. From Alkyl halides:
- Alkyl halides (R-X) on heating with alcoholic potash, eliminate one molecule of hydrogen halide to form alkenes.
- This reaction is known as dehydrohalogenation i.e., removal of hydrogen halide.
- Since hydrogen atom is eliminated from the β carbon atom, the reaction is also known as β-elimination reaction.
CH3-CH2X → CH2 = CH2 + HX
CH3-CH2-CH2Br → CH3-CH = CH2 + HBr
3. From vicinal dihalides:
- Dihalides in which two halogen atoms are attached to two adjacent carbon atoms are known as vicinal (vic) dihalides.
- Vicinal dihalides on treatment with zinc metal lose a molecule of ZnX2 to form an alkene.
- This reaction is known as dehalogenation (i.e. elimination of halogen molecule).
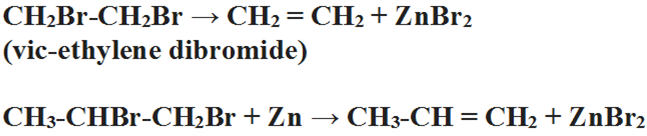
4. From alcohols:
- Alcohols when heated with concentrated sulphuric acid undergo dehydration (elimination of water molecule) to form alkenes.
- This reaction is also the example of β-elimination reaction since –OH group is eliminated from the β-carbon atom.
CH3-CH2-OH → CH2 = CH2 + H2O
Chemical Properties
1.Addition of hydrogen:
Alkenes add hydrogen in presence of finely divided Ni, Pd or Pt to form alkanes.
CH2 = CH2 + H2 → CH3-CH3
2. Addition of halogen:
Alkenes add halogen (Cl2 orBr2) to form vicinal dihalides.
CH2 = CH2 + X2 → CH2X-CH2X
When Br2 dissolved in CCl4 (carbon tetrachloride) is added to unsaturated compounds (alkenes or alkynes), the reddish orange colour of bromine solution is discharged. This reaction is used as a test for unsaturation.
CH2 = CH2 + Br2 → CH2Br-CH2Br
3. Addition of hydrogen halide:
Alkenes add hydrogen halide to form alkyl halides.
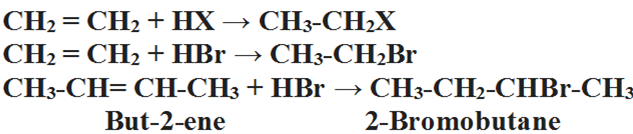
Addition of HBr to unsymmetrical alkenes (Markovnokov’s Rule)
When HBr is added to propene, we get 2 products – 1-bromopropane and 2-bromopropane.
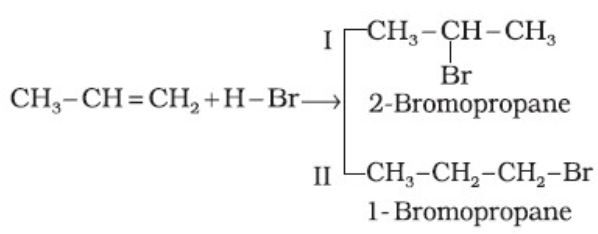
Here the major product is determined by a rule called Markovnikov rule (Markownikoff’s rule). The rule states that “when an unsymmetrical reagent is added to an unsymmetrical alkene, the negative part of the addendum (adding molecule) gets attached to the carbon containing lesser number of hydrogen atoms” (less hydrogenated C atom).
Thus in the above reaction 2-bromopropane is the major product.
In the presence of peroxide, addition of HBr to unsymmetrical alkenes takes place against Markovnikov rule. This is known as peroxide or Kharash effect or anti-Markovnikov addition reaction.
e.g.
![]()
4. Addition of water:
Alkenes add water in presence of a few drops of concentrated sulphuric acid to form alcohols. The reaction occurs according to the Markovnikov rule.
![]()
5. Oxidation:
(i) When oxidised using cold and dilute aqueous solution of potassium permanganate (KMnO4) [commonly called Baeyer’s reagent], alkenes give vicinal glycols.
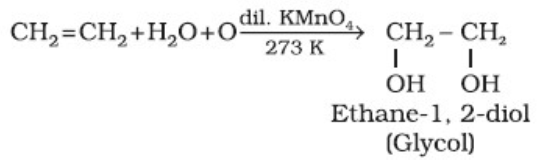
When KMnO4 is added to alkene, its pink colour gets discharged. So this reaction is also used as a test for unsaturation.
(ii) Acidic KMnO4 or acidic potassium dichromate (K2Cr2O7) oxidises alkenes to ketone or acids depending on the nature of the alkene.
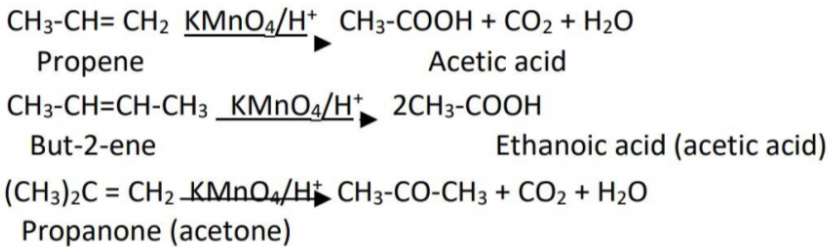
6. Ozonolysis: Alkenes add ozone to form an ozonide which on hydrolysis in presence of Zn to form carbonyl compounds (Aldehydes or ketones).
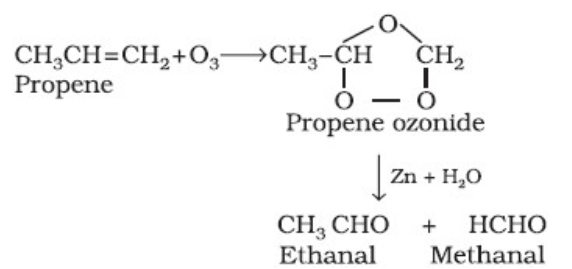
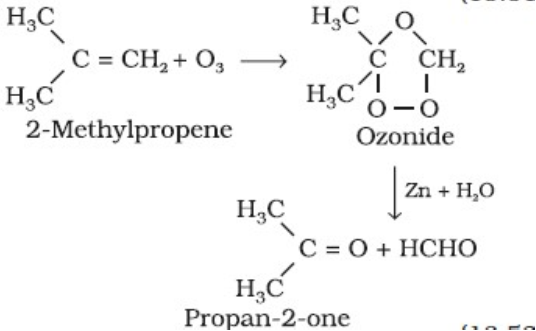
7. Polymerisation:
- The complex organic molecules formed by the combination of simple molecules are called polymers (macromolecules) and the reaction is called polymerisation reaction.
- The simple molecule from which a polymer is formed is called monomer.
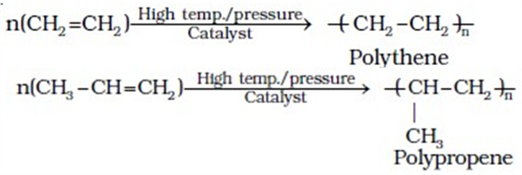

 Ritan Sheth
Ritan Sheth