- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
SOME COMPOUNDS OF BORON
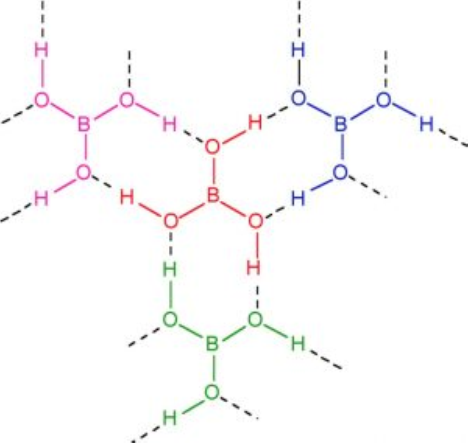
Structure of boric acid
It has a layer structure in which planar BO3 units are joined by hydrogen bonds as shown in Fig.
Physical properties of boric acid:
(i) It is a white crystalline solid.
(ii) It is soft soapy in touch.
(iii) It is sparingly soluble in cold water but fairly soluble in hot water.
Uses:
(i) In the manufacture of heat resistant borosilicate glazes.
(ii) As a preservative for milk and food stuffs.
(iii) In the manufacture of enamels and glazes in pottery.
(iii) Diborane, (B2H6): The series of compounds of boron with hydrogen is known as boranes.
Diborane is prepared by the reduction of boron trifluoride with LiAlH4 in diethyl ether.
4BF3 + 3LiAlH4 ——> 2B2H6+ 3LiF + 3AlF3
Laboratory method of preparation. In laboratory diborane is prepared by the oxidation of sodium borohydride with iodine.
2NaBH4 + I2 ——> B2H6 + 2NaI +H2
Industrial method of preparation. On industrial scale, diborane is prepared by reduction of BF3 with sodium hydride.
![]()
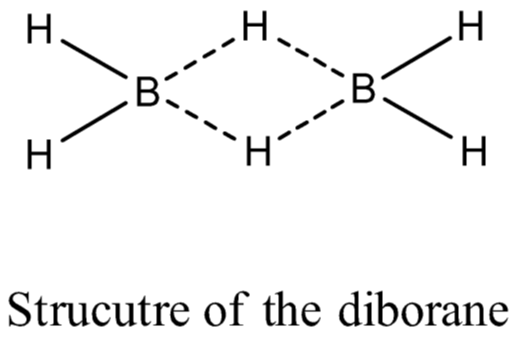
Physical Properties:
(i) Diborane is a colourless, highly toxic gas with a b.p. of 180 K.
(ii) Diborane catches fire spontaneously upon exposure to air.
(iii) Higher boranes are spontaneously flammable in air.
Chemical properties:
(i) Boranes are readily hydrolysed by water to form boric acid
B2H6(g) + 6H20(Z) ——> 2B(OH)3(aq) + 6H2(g)
(ii) It burns in oxygen evolving an enormous amount of heat
B2H6 + 302 —–> B203 + 3H20
(iii) Reaction with Lewis base:
Diborane on treatment with lewis bases undergo cleavage reactions to form borane which then reacts with Lewis bases to form adducts.
B2H6 + 2NMe3 ——> 2BH3. NMe3
B2H6 + 2CO ———> 2BH3 .CO

 Ritan Sheth
Ritan Sheth
