- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
PURIFICATION OF ORGANIC COMPOUNDS
An oganic compound may contain impurities and is essential to purify it. Various methods used for the purification of organic compounds are based on the nature of the compound and the impurity present in it.
The common techniques used for purification are as follows:
- Sublimation
- Crystallisation
- Distillation
- Differential extraction and
- Chromatography
1. Sublimation
- It is the process of conversion of a solid substance directly to vapour by heating.
- It is used to separate sublimable compounds from non-sublimable impurities.
- In this method, the substance is placed in a sublimation apparatus and heated under vacuum.
- Under this reduced pressure, the solid sublimes and condenses as a purified compound on a cooled surface.
- The impurities left behind on the apparatus.
- This method is used for the purification of naphthalene, iodine, camphor etc.
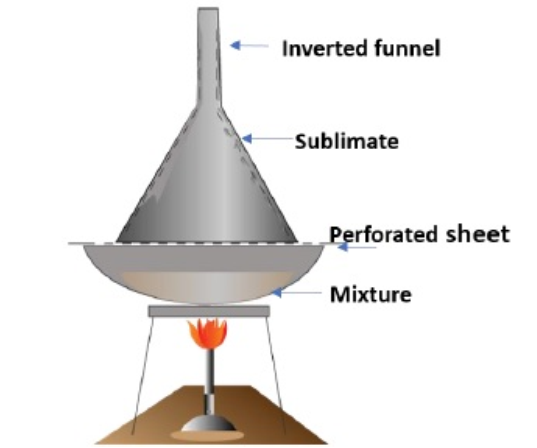
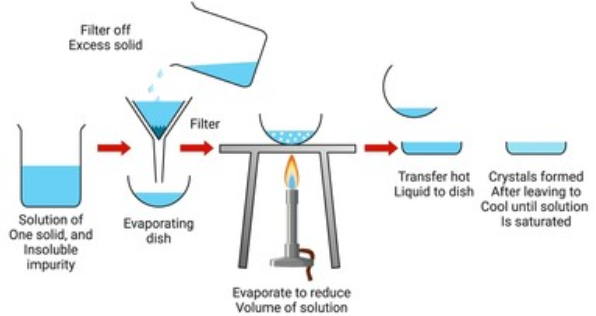
2. Crystallisation
- This is one of the most commonly used techniques for the purification of solid organic compounds.
- It is based on the difference in the solubilities of the compound and the impurities in a suitable solvent.
- The impure compound is dissolved in a solvent in which it is sparingly soluble at room temperature but appreciably soluble at higher temperature.
- The solution is concentrated to get a nearly saturated solution.
- On cooling the solution, pure compound crystallises out and is removed by filtration.
- If the compound is highly soluble in one solvent and very little soluble in another solvent, crystallisation can be satisfactorily carried out in a mixture of these solvents.
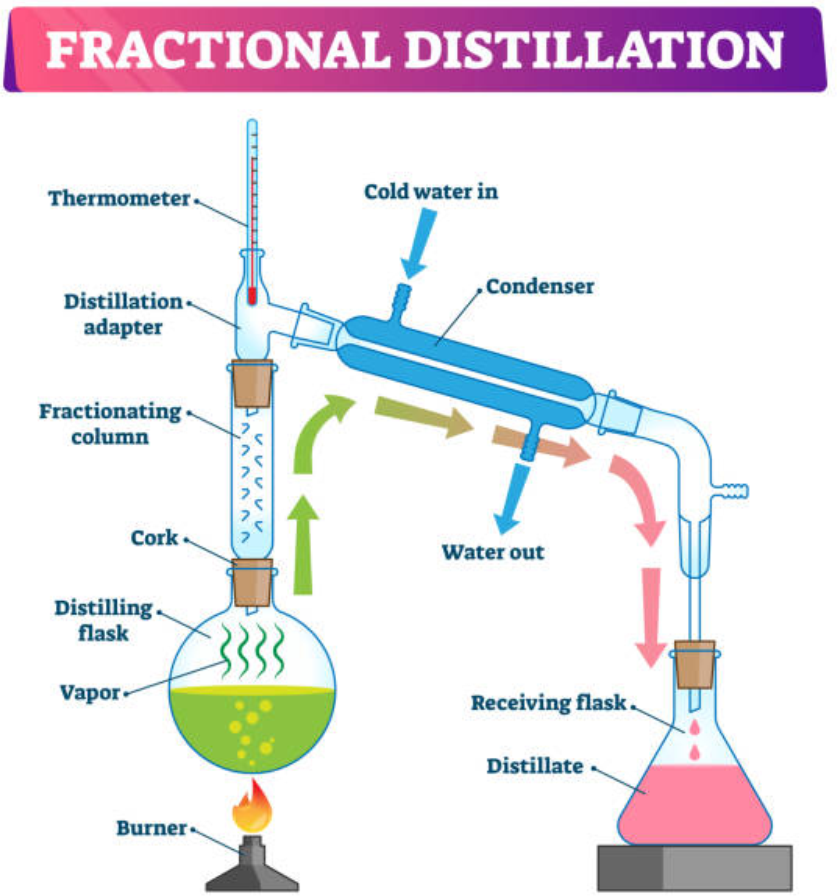
3. Distillation
This method is used to separate
(i) volatile liquids from non-volatile impurities and
(ii) the liquids having sufficient difference in their boiling points.
- The principle of this method is that liquids having different boiling points vaporise at different temperatures.
- The vapours are cooled and the liquids so formed are collected separately.
- In this method, the liquid mixture is taken in a round bottom flask and heated carefully.
- On boiling, the vapours of lower boiling liquid are formed first.
- The vapours are condensed by using a condenser and the liquid is collected in a receiver.
- The vapours of higher boiling liquid form later and it can be collected separately.
- Chloroform (b.p 334 K) and aniline (b.p. 457 K) are separated by this technique.
There are different types of distillation methods. They are:
a) Fractional distillation:
- Fractional distillation is used to separate two or more liquids that are miscible.
- It is a special type of distillation designed to separate a mixture of two or more liquids that have different boiling points.
- The process involves heating the mixture and partial condensation of the vapours along a fractionating column.
- The column is set up such that components with lower boiling points pass through the column and are collected earlier than components with higher boiling points.
- Repeated vaporization and condensation result in the separation of the components of the mixture.
- The efficiency of fractional distillation depends on the use of the fractionating column.
- The fractionating column is packed with glass beads.
- It provides a large surface area for vaporization and condensation of the liquid mixture.
- Ethanol and water, crude oil, toluene and cyclohexane etc are separated by this method.
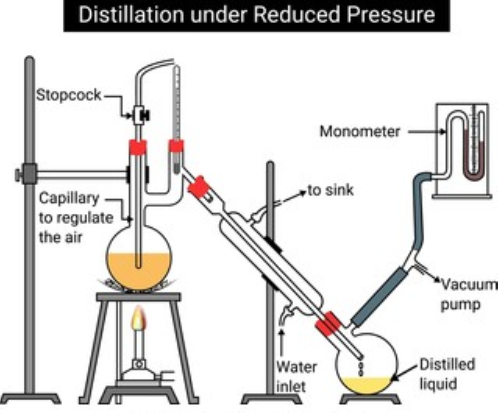
b) Distillation under reduced pressure:
- This method is used to purify liquids having very high boiling points and those, which decompose at or below their boiling points.
- Such liquids are made to boil at a temperature lower than their normal boiling points by reducing the pressure on their surface.
- The pressure is reduced with the help of a water pump or vacuum pump.
- Glycerol can be separated from spent-lye in soap industry by using this technique.
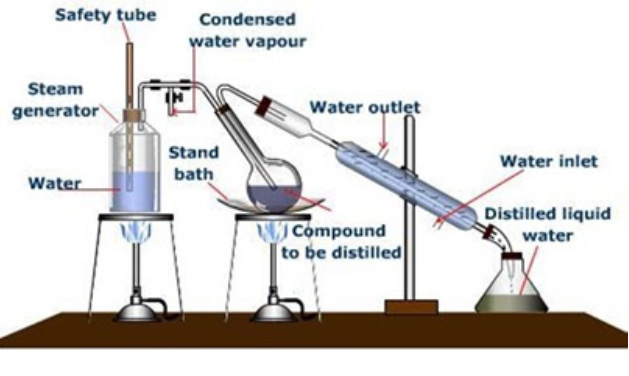
c) Steam Distillation:
- This technique is applied to separate substances which are steam volatile and are immiscible with water.
- In steam distillation, steam from a steam generator is passed through a heated flask containing the liquid to be distilled.
- The mixture of steam and the volatile organic compound is condensed and collected.
- The compound is later separated from water using a separating funnel.
- Aniline – water mixture is separated by this method.
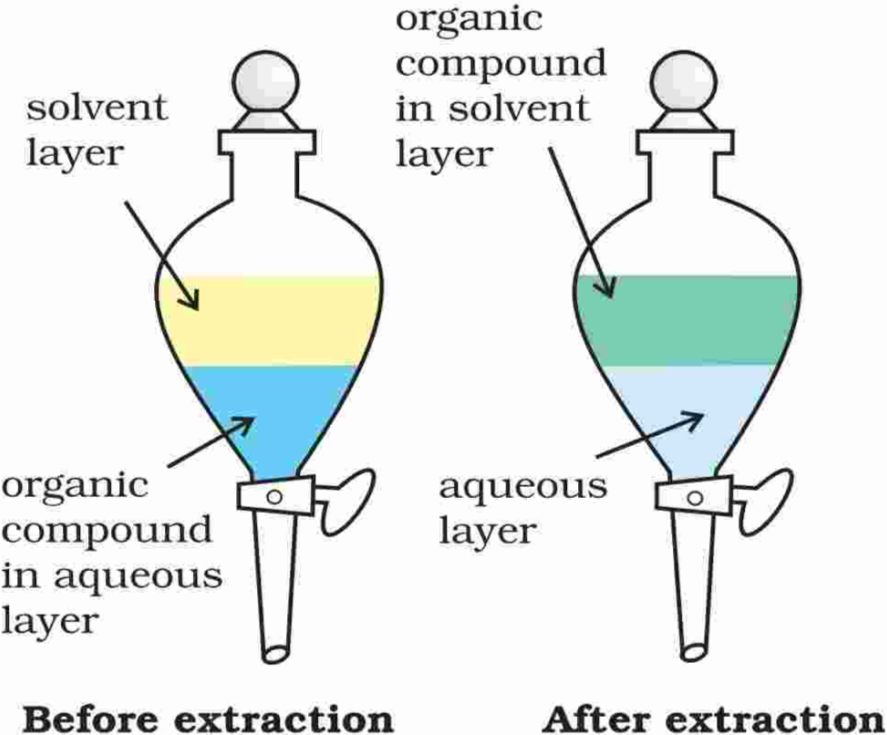
4. Differential Extraction
- When an organic compound is present in an aqueous medium, it is separated by shaking it with an organic solvent in which it is more soluble than in water.
- The organic solvent and the aqueous solution should be immiscible with each other.
- So they form two distinct layers which can be separated by separating funnel.
- The organic solvent is later removed by distillation or by evaporation to get back the compound
5. Chromatography
- This method is used to separate mixtures into their components, to purify compounds and to test the purity of compounds.
- Here the mixture to be separated is passed through a stationary phase, which may be a solid or a liquid.
- A pure solvent (sometimes a mixture of solvents or a gas) is allowed to move slowly over the stationary phase.
- The moving phase is called the mobile phase.
- The components of the mixture get gradually separated from one another.
Based on the principle involved, there are mainly two types of chromatography:
- Adsorption chromatography, and
- Partition chromatography
a) Adsorption Chromatography
Adsorption chromatography is based on the fact that different compounds are adsorbed on an adsorbent in different degrees. Commonly used adsorbents are silica gel and alumina.
- Here a mobile phase is allowed to move over a stationary phase (adsorbent).
- Based on the adsorbing power, the components of the mixture are adsorbed at different places over the stationary phase.
- Following are two main types of chromatographic techniques based on the principle of differential adsorption.
-
- Column chromatography
- Thin layer chromatography.
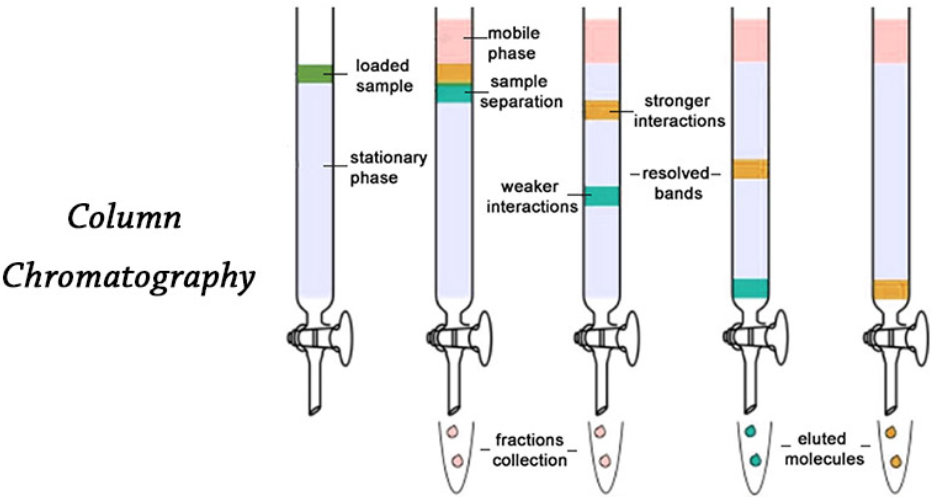
1. Column Chromatography:
- It involves the separation of a mixture over a column of adsorbent (stationary phase) packed in a glass tube.
- The column is fitted with a stopcock at its lower end. The mixture to be separated is passed through the column.
- Based on the adsorbing power, the components are adsorbed at different places over the column.
- The most readily adsorbed substances are retained near the top and others come down to various distances in the column.
- Then an appropriate eluant (solvent) is allowed to flow down the column slowly.
- Different solvents are used to separate different components.
- So the components can be collected separately and they can be separated.
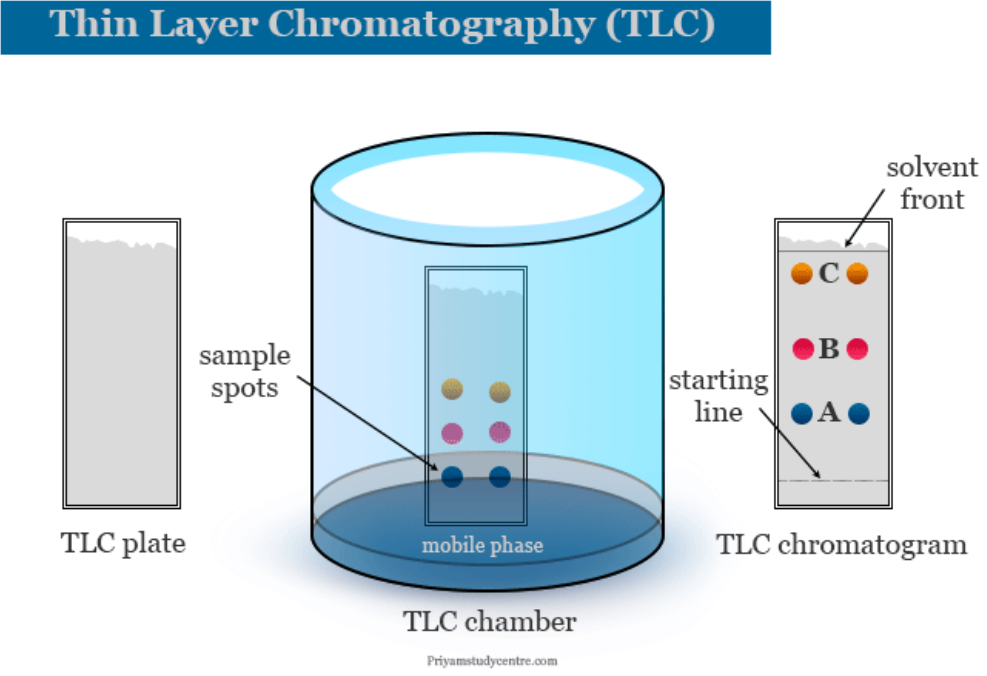
2. Thin Layer Chromatography (TLC):
- It is another type of adsorption chromatography. It involves separation of substances of a mixture over a thin layer of an adsorbent coated on a glass plate.
- A thin layer (about 0.2mm thick) of an adsorbent (silica gel or alumina) is spread over a glass plate of suitable size.
- The plate is known as thin layer chromatography plate or chromaplate.
- The solution of the mixture to be separated is applied as a small spot about 2 cm above one end of the TLC plate.
- The glass plate is then placed in a closed jar containing the eluant.
- As the eluant rises up the plate, the components of the mixture move up along with the eluant to different distances depending on their degree of adsorption and separation takes place.
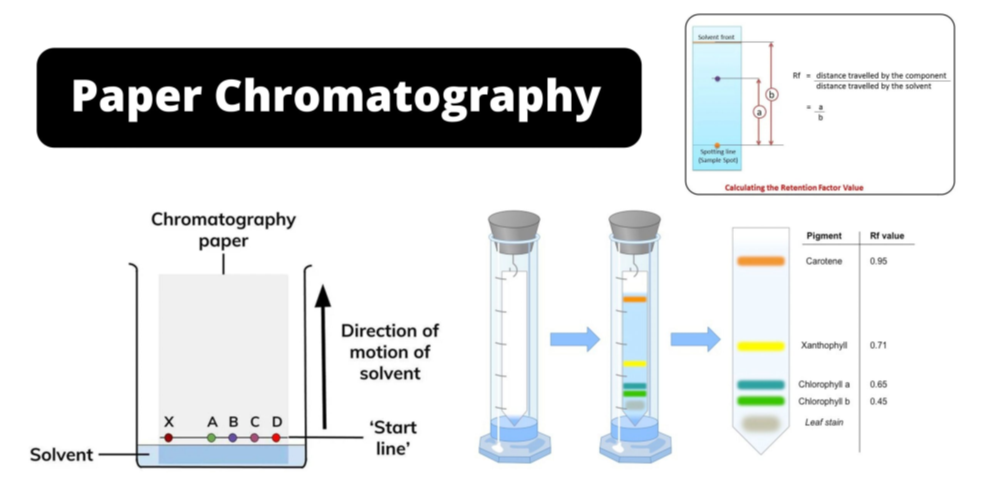
- The relative adsorption of each component of the mixture is expressed in terms of its retardation factor (Rf value).
Rf = Distance moved by the substance from base line (x) / Distance moved by the solvent from base line (y)
The spots of coloured compounds are visible on TLC plate due to their original colour. The spots of colourless compounds can be detected by putting the plate under ultraviolet light. Another detection technique is to place the plate in a covered jar containing a few crystals of iodine. Spots of compounds, which adsorb iodine, will show up as brown spots. Sometimes an appropriate reagent may also be sprayed on the plate.
b) Partition Chromatography:
- It is based on continuous differential partitioning of components of a mixture between stationary and mobile phases.
- Paper chromatography is a type of partition chromatography. In paper chromatography, a special quality paper known as chromatography paper is used.
- Chromatography paper contains water trapped in it, which acts as the stationary phase.
- A strip of chromatography paper, spotted at the base with the solution of the mixture, is suspended in a suitable solvent or a mixture of solvents.
- This solvent acts as the mobile phase.
- The solvent rises up the paper by capillary action and flows over the spot.
- The paper selectively retains different components according to their differing partition in the two phases.
- The paper strip is known as a chromatogram.
- The spots of the separated coloured compounds are visible at different heights from the position of initial spot on the chromatogram.
- These spots can be visible by u.v. light or by spraying suitable reagents.

 Ritan Sheth
Ritan Sheth
