- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
BOND PARAMETERS
• Bond Length
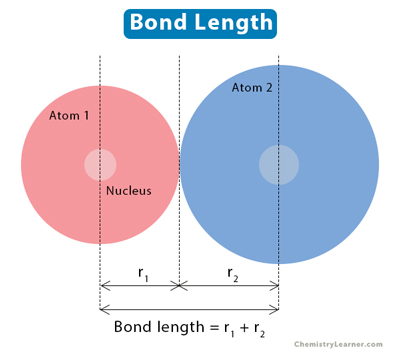
It is defined as the equilibrium distance between the centres of the nuclei of the two bonded atoms. It is expressed in terms of A. Experimentally, it can be defined by X-ray diffraction or electron diffraction method.
• Bond Angle
It is defined as -the angle between the lines representing the orbitals containing the bonding – electrons.
It helps us in determining the shape. It can be expressed in degree. Bond angle can be experimentally determined by spectroscopic methods.
• Bond Enthalpy
It is defined as the amount of energy required to break one mole of bonds of a particular type to separate them into gaseous atoms.
Bond Enthalpy is also known as bond dissociation enthalpy or simple bond enthalpy. Unit of bond enthalpy = kJ mol-1
Greater the bond enthalpy, stronger is the bond. For e.g., the H—H bond enthalpy in hydrogen is 435.8 kJ mol-1.
The magnitude of bond enthalpy is also related to bond multiplicity. Greater the bond multiplicity, more will be the bond enthalpy. For e.g., bond enthalpy of C —C bond is 347 kJ mol-1 while that of C = C bond is 610 kJ mol-1.
In polyatomic molecules, the term mean or average bond enthalpy is used.
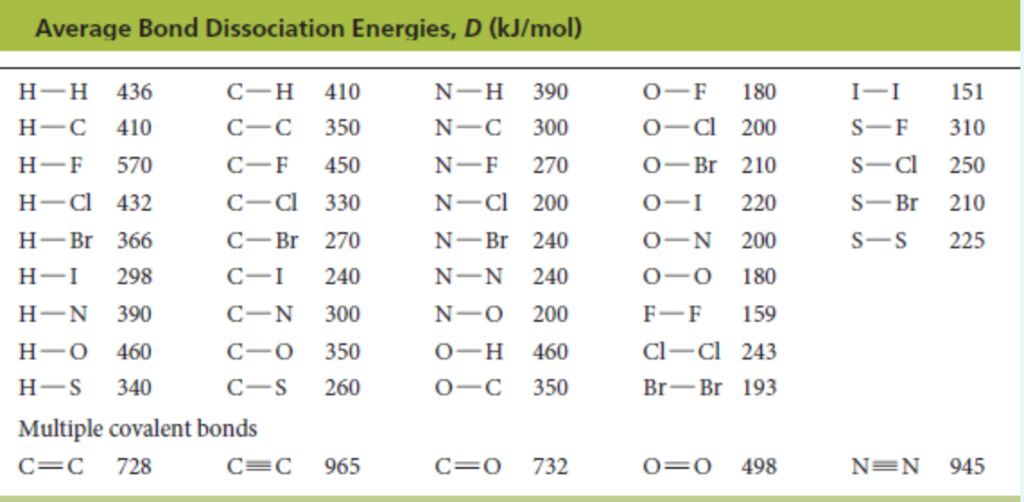
• Bond Order
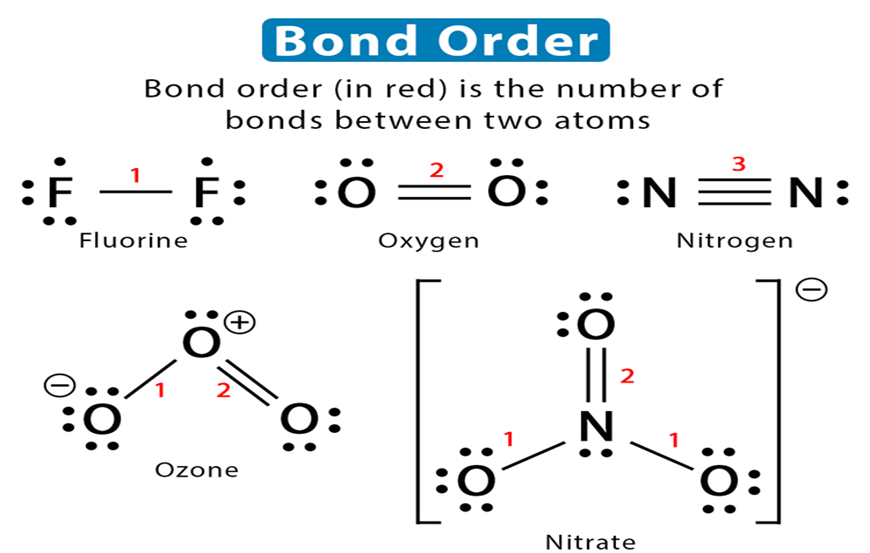
According to Lewis, in a covalent bond, the bond order is given by the number of bonds between two atoms in a molecule. For example,
Bond order of H2 (H —H) =1
Bond order of 02 (O = O) =2
Bond order of N2 (N = N) =3
Isoelectronic molecules and ions have identical bond orders. For example, F2 and O22- have bond order = 1. N2, CO and NO+ have bond order = 3. With the increase in bond order, bond enthalpy increases and bond length decreases. For example,
• Resonance Structures
There are many molecules whose behaviour cannot be explained by a single-Lew is structure, Tor example, Lewis structure of Ozone represented as follows:
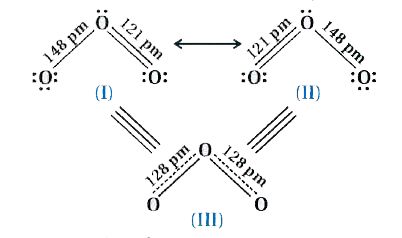
Thus, according to the concept of resonance, whenever a single Lewis structure cannot explain all the properties of the molecule, the molecule is then supposed to have many structures with similar energy.
Positions of nuclei, bonding and nonbonding pairs of electrons are taken as the canonical structure of the hybrid which describes the molecule accurately. For 03, the two structures shown above are canonical structures and the III structure represents the structure of 03 more accurately. This is also called resonance hybrid.
Some resonating structures of some more molecules and ions are shown as follows:
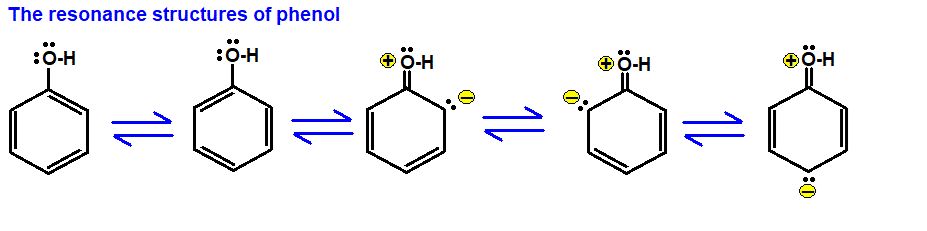
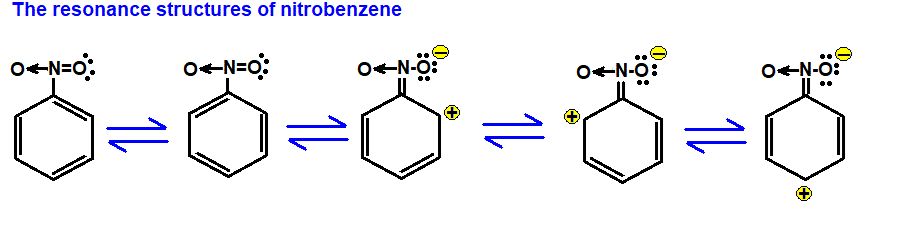
• Polarity of Bonds
Polar and Non-Polar Covalent bonds
Non-Polar Covalent bonds: When the atoms joined by covalent bond are the same like; H2, 02, Cl2, the shared pair of electrons is equally attracted by two atoms and thus the shared electron pair is equidistant to both of them.
Alternatively, we can say that it lies exactly in the centre of the bonding atoms. As a result, no poles are developed and the bond is called as non-polar covalent bond. The corresponding molecules are known as non-polar molecules.
For Example,
![]()
![]()
Polar bond: When covalent bonds formed between different atoms of different electronegativity, shared electron pair between two atoms gets displaced towards highly electronegative atoms.
For Example, in HCl molecule, since electronegativity of chlorine is high as compared to hydrogen thus, electron pair is displaced more towards chlorine atom, thus chlorine will acquire a partial negative charge (δ–) and hydrogen atom have a partial positive charge (δ+) with the magnitude of charge same as on chlorination. Such covalent bond is called polar covalent bond.
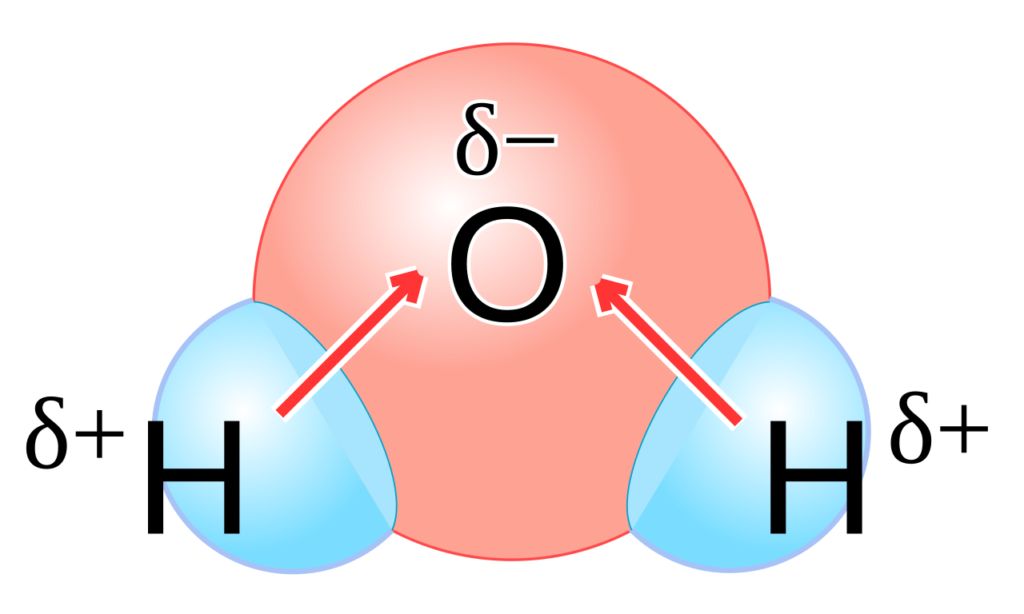
• Dipole Moment
Due to polarity, polar molecules are also known as dipole molecules and they possess dipole moment. Dipole moment is defined as the product of magnitude of the positive or negative charge and the distance between the charges.
Dipole Moment (μ) = Charge (Q) x (d) (Distance of Separation)
It is expressed in Debye units (D)
1D= 3.33564 x 10-30 cm
Dipole moment is a vector quantity. It is depicted by a small arrow with tail on positive center and head pointing towards the negative centre. For example, H – F
With the help of dipole moment the degree of polarity of bonds can be expressed.
• Applications of Dipole Moment
(i) For determining the polarity of the molecules.
(ii) In finding the shapes of the molecules.
For example, the molecules with zero dipole moment will be linear or symmetrical. Those molecules which have unsymmetrical shapes will be either bent or angular.
(e.g., NH3with μ = 1.47 D).
(iii) In calculating the percentage ionic character of polar bonds.

 Ritan Sheth
Ritan Sheth
