- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
DALTON’S ATOMIC THEORY
In 1808, Dalton published ‘A New System of Chemical Philosophy’ in which he proposed the following:
1. Matter consists of indivisible atoms.
2. All the atoms of a given element have identical properties including identical mass. Atoms of different elements differ in mass.
3. Compounds are formed when atoms of different elements combine in a fixed ratio.
4. Chemical reactions involve reorganisation of atoms. These are neither created nor destroyed in a chemical reaction.
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Chemistry
DALTON LAW OF PARTIAL PRESSURE
At a given temperature, the total pressure exerted by two or more non-reacting gases occupying a definite volume is equal to the sum of the partial pressures of the component gases. Mathematically,
P = pA + pB + pC + …..
when P is the total pressure and pA, pB, pC, ….. are the partial pressures of the component gases A, B, C, ….. respectively. The pressure that a component gas of the gaseous mixture would exert if it were only present in the volume under consideration at a given temperature, is the partial pressure of the component.
Derivation of Dalton’s Law
Let n1 and n2 be the no. of moles of two non-reacting gases ‘A’ and ‘B’ filled in a vessel of volume ‘V’ at temperature T.
Total pressure in the vessel ‘P’ may be calculated as
PV = (n1 + n2)RT …….(i)
Individual or partial pressure may be calculated as,
pAV = n1RT …….(ii)
pBV = n2RT …….(iii)
Adding (ii) and (iii), we get
(pA + pB)V = (n1 + n2)RT …….(iv)
Comparing equations (i) and (iv), we get
P = pA + pB (Dalton’ expression)
Dividing equation (ii) by (i), we get
![]() = xA
= xA
pA = xA ´ P
where xA = mole fraction of ‘A’. Similarly, dividing (iii) by (i), we get
pB = xB ´ P
i.e., Partial pressure of a component = Mole fraction ´ total pressure
Relationship between total pressure and individual pressures (before mixing) of the constituent gases at constant temperature
At constant temperature, let V1 volume of a gas A at a pressure p1 be mixed with V2 volume of gas B at a pressure p2. Both these gases do not react chemically.
Total volume = V1 + V2
Let the total pressure be P and partial pressures of A and B be pA and pB respectively. Applying Boyle’s law,
PA(V1 + V2) = p1V1 …….(i)
and pB(V1 + V2) = p2V2 …….(ii)
Adding (i) and (ii)
pA + pB = 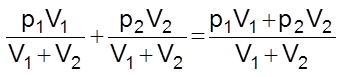
or P = 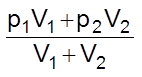
Dalton’s Law fails in those gases which react chemically so not applicable to the following mixtures.
(i) NH3+HCl
(ii) NO +O2
(iii) H2+F2
(iv) H2+Cl2
(v) SO2+ Cl2
Applications
(i) In the determination of pressure of dry gas : When a gas is called over water, then it is moist , water also vaporizes simultaneously and exerts its own partial pressure. Partial pressure of water is called its aqueous tension. It depends upon temperature.
If P and P¢ are the pressure of the dry gas and the moist gas respectively at t°C and p is the aqueous tension at that temperature, then by Dalton’s Law of Partial Pressures
P = P¢ - p
(ii) In the calculation of partial pressures : In a mixture of non-reacting gases A, B, C etc., if each gas is considered to be an ideal gas, then applying PV = nRT
PA = nA ![]() , pB = nB
, pB = nB ![]() pC = nC
pC = nC ![]()
And so on.
By Dalton’s law of partial pressures,
Total pressure, P = pA + pB + pC + … = ![]() (nA + nB + nC + …)
(nA + nB + nC + …)
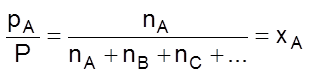 (mole fraction of A)
(mole fraction of A)
or pA = xA ´ P
Similarly, pB = xB ´ P and so on. Thus
Partial pressure of A = Mole fraction of A ´ Total pressure
Amagat Law of Partial volume
Total volume of a mixture of gases which do not react at constant temperature and pressure is equal to sum of individual volumes (partial volumes) of constituent gases.
V = åVi = V1 + V2 + V3 + ….. + Vn

 Ritan Sheth
Ritan Sheth
