- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ALLOTROPES OF CARBON
The property of an element to exist in two or more forms which have different physical properties but identical chemical properties is called allotropy and different forms are called allotropes. Carbon exists in two allotropic forms:
(i) Crystalline
(ii) Amorphous
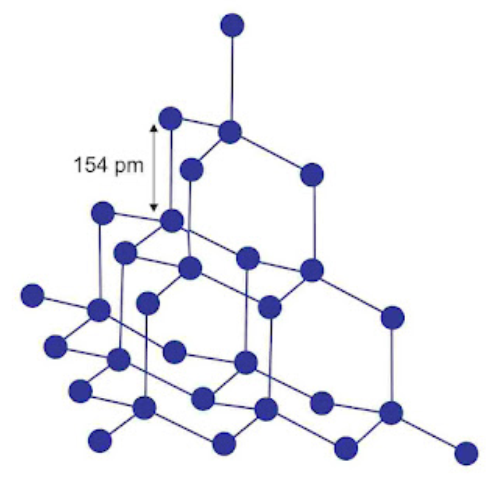
Crystalline form of carbon: Diamond, Graphite, Fullerenes Diamond: In diamond each carbon atom undergoes sp3 hybridisation. Each carbon is tetrahedrally linked to four other carbon atoms. The C—C bond length is 154 pm.
Properties:
(i) It is the hardest substance on earth.
(ii) It is used as an abrasive for sharpening hard tools in making dyes and in manufacture of tungsten filaments.
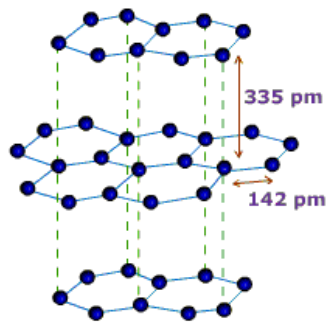
Graphite: In graphite, carbon is sp2-hybridized. Graphite has a two-dimensional sheet like structure consisting of a number of hexagonal rings fused together. Layers are held by van der Waals forces and distance between two layers is 340 pm.
Properties:
(i) Graphite conducts electricity along the sheet.
(ii) It is very soft and slippery.
(iii) Used as a dry lubricant in machines running at high temperature, where oil cannot be used as a lubricant.
Fullerenes: Fullerenes was discovered collectively by three scientists namely E. Smalley, R.F. Curl and H.W. Kroto.
Preparation:
Fullerenes is prepared by heating of graphite in an electric arc in the presence of inert gas such as helium or argon.
The sooty material formed by the condensation of vapourised Cn small molecules consists of mainly with smaller quantity of C70 and traces of other fullerenes consisting of even number of carbon atoms up to 350or above.
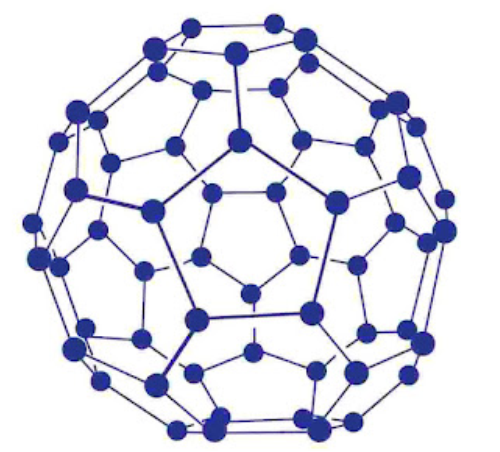
Fullerenes are cage like molecules. C60 molecule has a shape like soccer ball and called Buckminsterfullerene’s. It is the most stable.
It contains 20 six-membered rings and 12 five-membered rings.
Six-membered rings are fused to both the other six-membered rings and five-membered rings but the five-membered rings are connected only to six-membered rings.
All the carbon atoms are equal and they undergo sp2-Kybridization.
Properties:
(i) Fullerenes being covalent are soluble in organic solvents.
(ii) It also forms platinum complexes.
Amorphous allotropic forms of carbon coke: It is a greyish black hard solid and is obtained by destructive distillation.
Wood charcoal: It is obtained by strong heating of wood in a limited supply of air.
Animal charcoal: It is obtained by the destructive distillation of bones.
Uses of carbon:
(i) Graphite fibre are used for making superior sports goods such as tennis and badminton rackets, fishing rods.
(ii) Being good conductor graphite is used for making electrodes for batteries and industrial electrolysis.
(iii) Being highly porous, activated charcoal is used for absorbing poisonous gases in gas masks. It is used to decolourize sugar.
(iv) Carbon black is used as black pigment in black ink and as filler in automobile tyres.
(v) Coke is extensively used as reducing agent in metallurgy.
(vi) Diamond is a precious stone.

 Ritan Sheth
Ritan Sheth
