- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
UNCERTAINTY IN MEASUREMENT
All scientific measurements involve certain degree of error or uncertainty. The errors which arise depend upon two factors.
(i) Skill and accuracy of the worker
(ii) Limitations of measuring instruments.
• Scientific Notation
It is an exponential notation in which any number can be represented in the form N x 10n where n is an exponent having positive or negative values and N can vary between 1 to 10. Thus, 232.508 can be written as 2.32508 x 102in scientific notation.
Now let us see how calculations are carried out with numbers expressed in scientific notation.
(i) Calculation involving multiplication and division
(a) (5.7×106) × (4.2×105) = (5.7×4.2) (106+5) = 23.94 × 1011
(b) (5.7×106) ÷ (4.2×103)
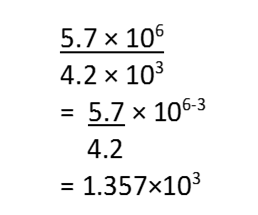
(ii) Calculation involving addition and subtraction: For these two operations, the first numbers are written in such a way that they have the same exponent. After that, the coefficients are added or subtracted as the case may be. For example,
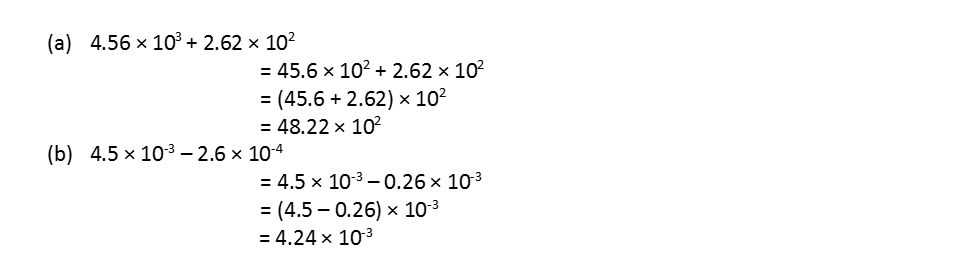
Significant Figures
Significant figures are meaningful digits which are known with certainty. There are certain rules for determining the number of significant figures. These are stated below:
1. All non-zero digits are significant. For example, in 285 cm, there are three significant figures and in 0.25 mL, there are two significant figures.
2. Zeros preceding to first non-zero digit are not significant. Such zeros indicates the position of decimal point.
For example, 0.03 has one significant figure and 0.0052 has two significant figures.
3. Zeros between two non-zero digits are significant. Thus, 2.005 has four significant figures.
4. Zeros at the end or right of a number are significant provided they are on the right side of the decimal point. For example, 0.200 g has three significant figures.
5. Counting numbers of objects. For example, 2 balls or 20 eggs have infinite significant figures as these are exact numbers and can be represented by writing infinite number of zeros after placing a decimal.
i.e., 2 = 2.000000
or 20 = 20.000000
• Addition and Subtraction of Significant Figures
In addition or subtraction of the numbers having different precisions, the final result should be reported to the same number of decimal places as in the term having the least number of decimal places.
For example, let us carry out the addition of three numbers 3.52, 2.3 and 6.24, having different precisions or different number of decimal places.
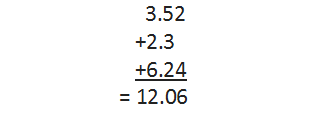
The final result has two decimal places but the answer has to be reported only up to one decimal place, i.e., the answer would be 12.0.
Subtraction of numbers can be done in the same way as the addition.

The final result has four decimal places. But it has to be reported only up to two decimal places, i.e., the answer would be 11.36.
• Multiplication and Division of Significant Figures
In the multiplication or division, the final result should be reported up to the same number of significant figures as present in the least precise number.
Multiplication of Numbers: 2.2120 x 0.011 = 0.024332
According to the rule the final result = 0.024
Division of Numbers: 4.2211÷3.76 = 1.12263
The correct answer = 1.12
• Dimensional Analysis
Often while calculating, there is a need to convert units from one system to other. The method used to accomplish this is called factor label method or unit factor method or dimensional analysis.

 Ritan Sheth
Ritan Sheth
