- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
BONDING IN SOME HOMONUCLEAR DIATOMIC MOLECULES
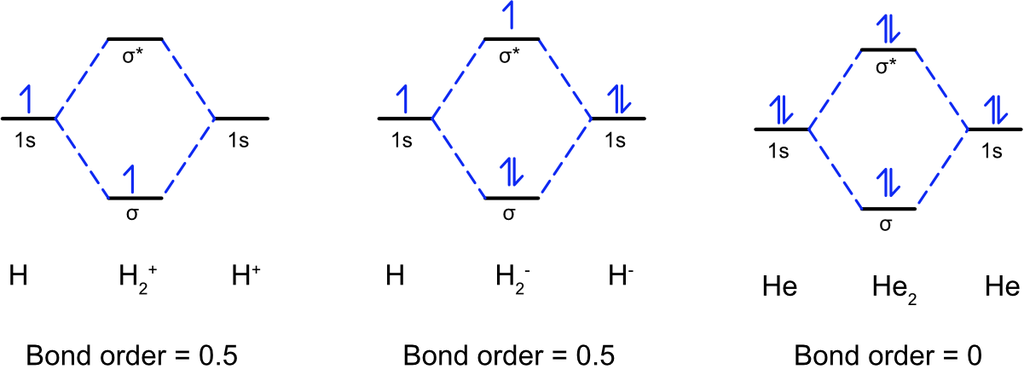
(1) Hydrogen molecule (H2): It is formed by the combination of two hydrogen atoms. Each hydrogen atom has one electron in Is orbital, so, the electronic configuration of hydrogen molecule is
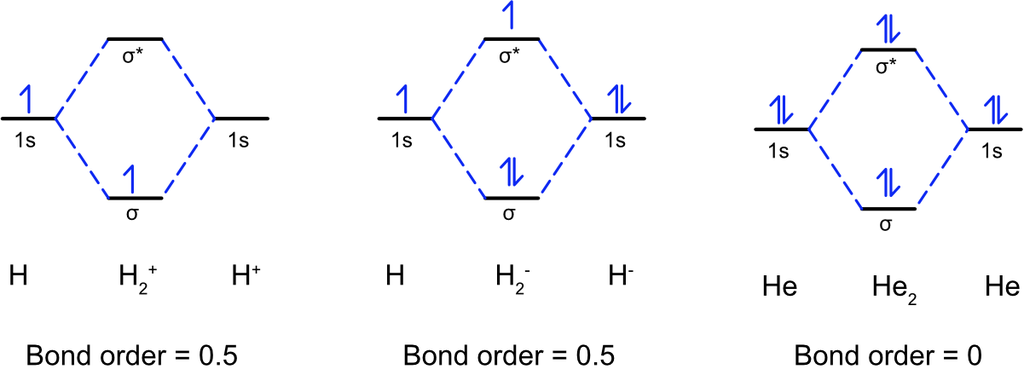
This indicates that two hydrogen atoms are bonded by a single covalent bond. Bond dissociation energy of hydrogen has been found = 438 kJ/mole. Bond-Length = 74 pm
No unpaired electron is present therefore,, it is diamagnetic.
(2) Helium molecule (He2): Each helium atom contains 2 electrons, thus in He2 molecule there would be 4 electrons.
The electrons will be accommodated in σ1s and σ*1s molecular orbitals:
Bond order (b.o.) = ½ (Nb–Na )
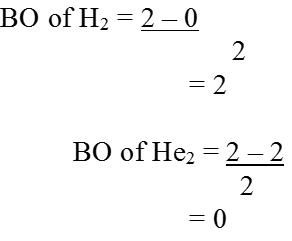

 Ritan Sheth
Ritan Sheth
