- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
APPLICATIONS
• Work (Pressure-volume Work)
Let us consider a cylinder which contains one mole of an ideal gas in which a frictionless piston is fitted.
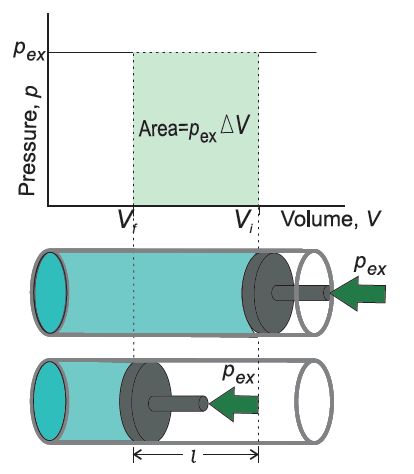
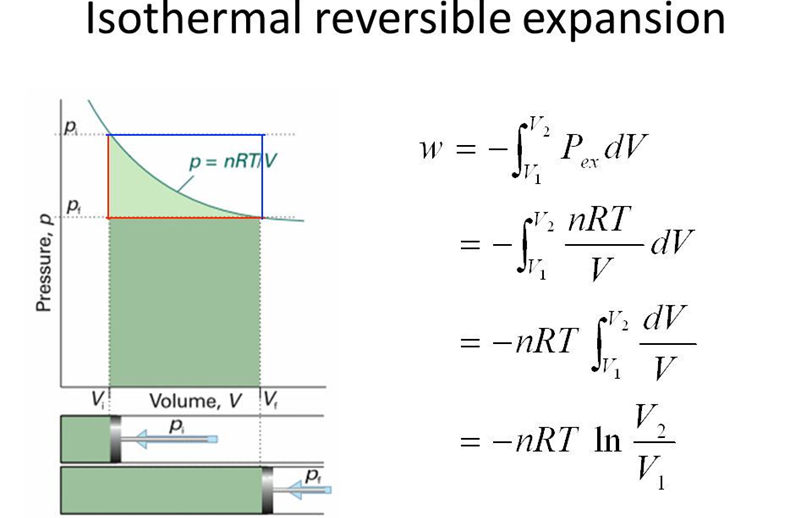
• Work Done in Isothermal and Reversible Expansion of Ideal Gas
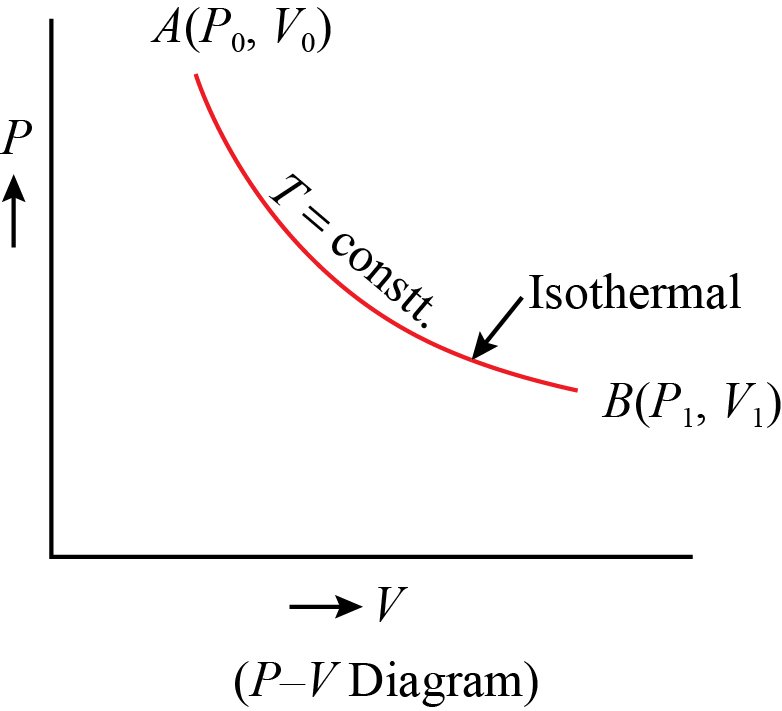
• Isothermal and Free Expansion of an Ideal Gas
For isothermal (T = constant) expansion of an
ideal gas into vacuum ; w = 0 since pex = 0.
Also, Joule determined experimentally that
q = 0; therefore, ∆U = 0
• Enthalpy (H)
It is defined as total heat content of the system. It is equal to the sum of internal energy and pressure-volume work.
Mathematically, H = U + PV
Change in enthalpy: Change in enthalpy is the heat absorbed or evolved by the system at constant pressure.
ΔH = qp
For exothermic reaction (System loses energy to Surroundings),
ΔH and qp both are -Ve.
For endothermic reaction (System absorbs energy from the Surroundings).
ΔH and qp both are +Ve.
Relation between ΔH and Δu.
![]() B
B
Let HA be the enthalpy of reactant A and HB be that of the products.
∴ HA = UA + PVA
HB = UB + PVB
ΔH = HB - HA
= (UB + PVB) – (UA + PVA)
ΔH = ΔU + PΔV (HB – HA)
ΔH = ΔU + PΔV
At constant pressure and temperature using ideal gas law,
PVA = nA RT (for reactant A)
PVB = nB RT (for reactant B)
Thus, PVB – PVA = nB RT - nA RT
= ( nB – nA) RT
PΔV = Δng RT
∴ ΔH = ΔU + Δng RT
• Extensive property
An extensive property is a property whose value depends on the quantity or size of matter present in the system.
For example: Mass, volume, enthalpy etc. are known as extensive property.
• Intensive property
Intensive properties do not depend upon the size of the matter or quantity of the matter present in the system.
For example: temperature, density, pressure etc. are called intensive properties.
• Heat capacity
The increase in temperature is proportional to the heat transferred.
q = coeff. x ΔT
q = CΔT
Where, coefficient C is called the heat capacity.
C is directly proportional to the amount of substance.
Cm = C/n
It is the heat capacity for 1 mole of the substance.
• Molar heat capacity
It is defined as the quantity of heat required to raise the temperature of a substance by 1° (kelvin or Celsius).
• Specific Heat Capacity
It is defined as the heat required to raise the temperature of one unit mass of a substance by 1° (kelvin or Celsius).
q = C x m x ΔT
where m = mass of the substance
ΔT = rise in temperature.
• Relation Between Cp and Cv for an Ideal Gas
At constant volume heat capacity = Cv
At constant pressure heat capacity = Cp
At constant volume qv= CvΔT = ΔU
At constant pressure qp = Cp ΔT = ΔH
For one mole of an ideal gas
ΔH = ΔU + Δ (PV) = ΔU + Δ (RT)
ΔH = ΔU + RΔT
On substituting the values of ΔH and Δu, the equation is modified as
Cp ΔT = CvΔT + RΔT
or Cp-Cv = R

 Ritan Sheth
Ritan Sheth
