- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
DEVELOPMENTS LEADING TO THE BOHR’S MODEL OF ATOM
Two developments played a major role in the formulation of Bohr’s model of atom. These were:
(i) Dual character of the electromagnetic radiation which means that radiations possess both wave like and particle like properties.
(ii) Experimental results regarding atomic spectra which can be explained only by assuming quantized electronic energy levels in atoms.
• Nature of Electromagnetic Radiation (Electromagnetic Wave Theory)
This theory was put forward by James Clark Maxwell in 1864. The main points of this theory are as follows:
(i) The energy is emitted from any source (like the heated rod or the filament of a bulb through which electric current is passed) continuously in the form of radiations and is called the radiant energy.
(ii) The radiations consist of electric and magnetic fields oscillating perpendicular to each other and both perpendicular to the direction of propagation of the radiation.
(iii) The radiations possess wave character and travel with the velocity of light 3 x 108 m/sec.
(iv) These waves do not require any material medium for propagation. For example, rays from the sun reach us through space which is a non-material medium.
• Characteristics of a Wave
Wavelength: It is defined as the distance between any two consecutive crests or troughs. It is represented by X and its S.I. unit is metre.
![]()
Frequency: Frequency of a wave is defined as the number of waves passing through a point in one second. It is represented by v (nu) and is expressed in Hertz (Hz).
1 Hz = 1 cycle/sec.
Velocity: Velocity of a wave is defined as the linear distance travelled by the wave in one second.
It is represented by c and is expressed in cm/sec or m/sec.
Amplitude: Amplitude of a wave is the height of the crest or the depth of the through. It is represented by V and is expressed in the units of length.
Wave Number: It is defined as the number of waves present in 1 metre length. Evidently it will be equal to the reciprocal of the wavelength. It is represented by bar v (read as nu bar).
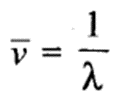
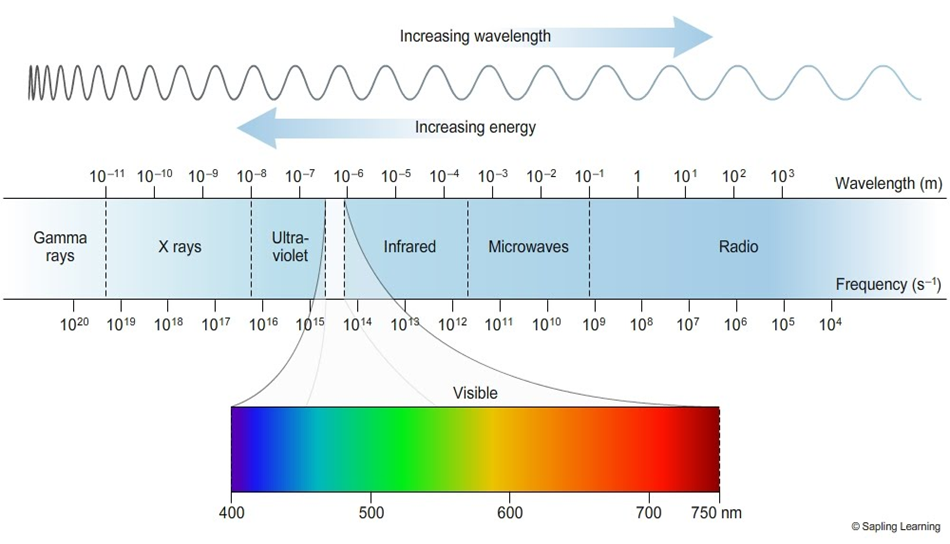
Electromagnetic Spectrum: When electromagnetic radiations are arranged in order of their increasing wavelengths or decreasing frequencies, the complete spectrum obtained is called electromagnetic spectrum.
![]()
• Limitations of Electromagnetic Wave Theory
Electromagnetic wave theory was successful in explaining properties of light such as interference, diffraction etc; but it could not explain the following:
(i) The phenomenon of black body radiation.
(ii) The photoelectric effect.
(iii) The variation of heat capacity of solids as a function of temperature.
(iv) The line spectra of atoms with reference to hydrogen.
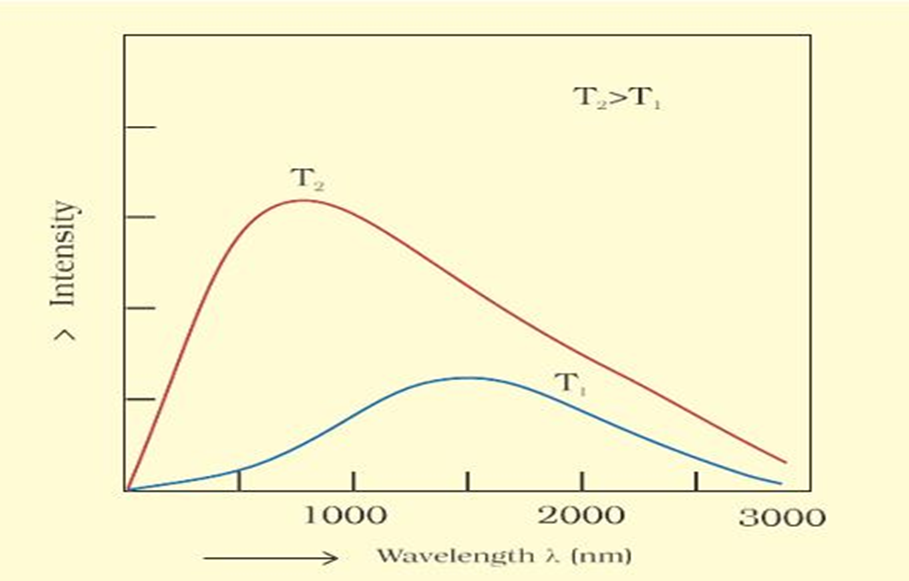
• Black Body Radiation
The ideal body, which emits and absorbs all frequencies is called a black body and the radiation emitted by such a body is called black body radiation. The. exact frequency distribution of the emitted radiation from a black body depends only on its temperature.
![]()
At a given temperature, intensity of radiation emitted increases with decrease of wavelength, reaches a maximum value at a given wavelength and then starts decreasing with further decrease of wavelength as shown in Fig 2.6.
• Planck’s Quantum Theory
To explain the phenomenon of ‘Black body radiation’ and photoelectric effect, Max Planck in 1900, put forward a theory known as Planck’s Quantum Theory.
This theory was further extended by Einstein in 1905. The main points of this theory was as follows: ,
(i) The radiant energy emitted or absorbed in the form of small packets of energy. Each such packets of energy is called a quantum.
(ii) The energy of each quantum is directly proportional to the frequency of the radiation
E ∝ ν
E = hν
where h is a proportionality constant, called Planck’s constant. Its value is equal to
6.626 x 10-34 J sec.
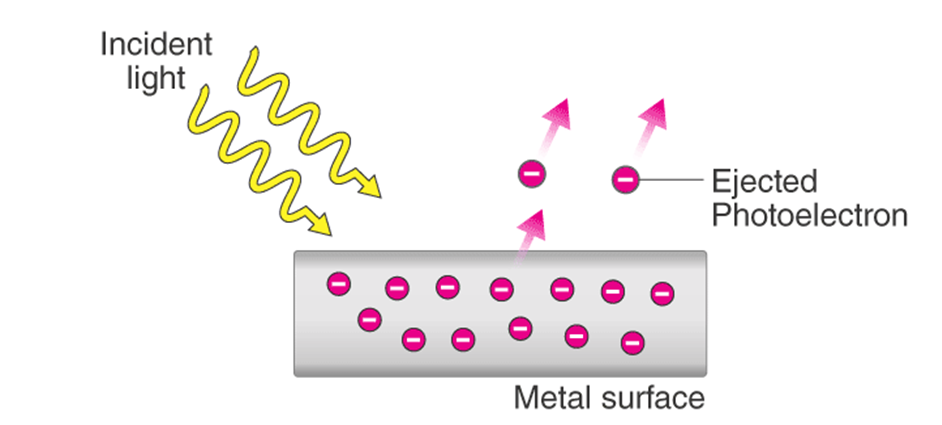
• Photoelectric Effect
Hertz, in 1887, discovered that when a beam of light of certain frequency strikes the surface of some metals, electrons are emitted or ejected from the metal surface. The phenomenon is called photoelectric effect.
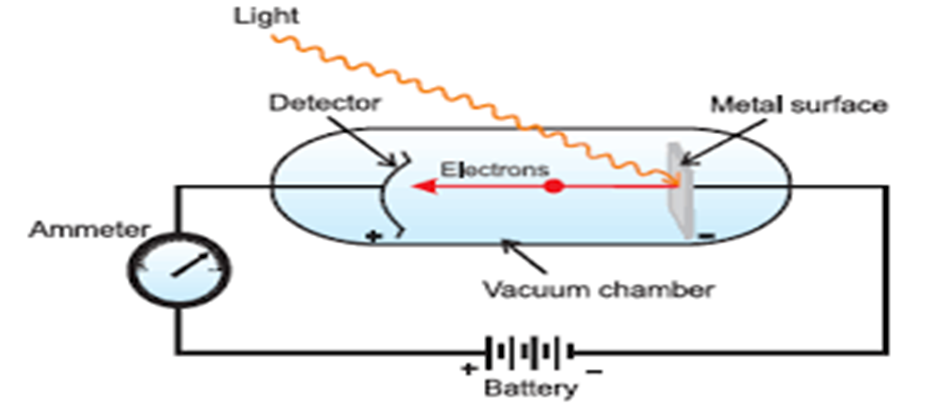
Observations in Photoelectric Effect
(i) Only photons of light of certain minimum frequency called threshold frequency (v0) can cause the photoelectric effect. The value of v0 is different for different metals.
(ii) The kinetic energy of the electrons which are emitted is directly proportional to the frequency of the striking photons and is quite independent of their intensity.
(iii) The number of electrons that are ejected per second from the metal surface depends upon the intensity of the striking photons or radiations and not upon their frequency.
Explanation of Photoelectric Effect
Einstein in (1905) was able to give an explanation of the different points of the photoelectric effect using Planck’s quantum theory as under:
(i) Photoelectrons are ejected only when the incident light has a certain minimum frequency (threshold frequency v0)
(ii) If the frequency of the incident light (v) is more than the threshold frequency (v0), the excess energy (hv – hv0) is imparted to the electron as kinetic energy.
K.E. of the ejected electron
energy of the emitted electron.
(iii) On increasing the intensity of light, more electrons are ejected but the energies of the electrons are not altered.
• Dual Behaviour of Electromagnetic Radiation
From the study of behaviour of light, scientists came to the conclusion that light and other electromagnetic radiations have dual nature. These are wave nature as well as particle nature. Whenever radiation interacts with matter, it displays particle like properties in contrast to the wavelike properties (interference and diffraction) which it exhibits when it propagates. Some microscopic particles, like electrons, also exhibit this wave-particle duality.
• Spectrum
When a ray of white light is passed through a prism the wave with shorter wavelength bends more than the one with a longer wavelength. Since ordinary white light consists of waves with all the wavelengths in the visible range, array of white light is spread out into a series of coloured bands called spectrum. The light of red colour which has longest wavelength is deviated the least while the violet light, which has shortest wavelength is deviated the most.
Continuous Spectrum
When a ray of white light is analysed by passing through a prism it is observed that it splits up into seven different wide bands of colours from violet to red (like rainbow). These colours are so continuous that each of them merges into the next. Hence, the spectrum is called continuous spectrum.
Emission Spectra
Emission Spectra is noticed when the radiations emitted from a source are passed through a prism and then received on the photographic plate. Radiations can be emitted in a number of ways such as:
(i) from sun or glowing electric bulb.
(ii) by passing electric discharge through a gas at low pressure.
(iii) by heating a substance to high temperature.
Line Spectra When the vapours of some volatile substance are allowed to fall on the flame of a Bunsen burner and then analysed with the help of a spectroscope. Some specific coloured lines appear on the photographic plate which are different for different substances. For example, sodium or its salts emit yellow light while potassium or its salts give out violet light.
Absorption Spectra
When white light is passed through the vapours of a substance and the transmitted light is then allowed to strike a prism, dark lines appear in the otherwise continuous spectrum. The dark lines indicate that the radiations corresponding to them were absorbed by the substance from the white light. This spectrum is called absorption spectrum.
Dark lines appear exactly at the same positions where the lines in the emission spectra appear.
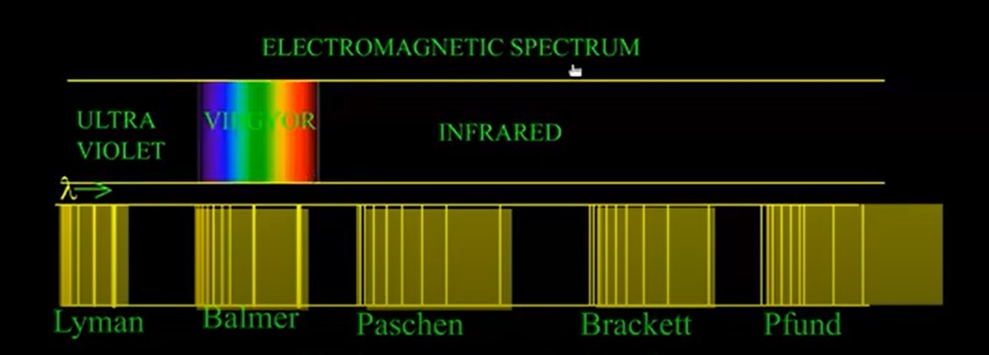
• Line Spectrum of Hydrogen
When electric discharge is passed through hydrogen gas enclosed in discharge tube under low pressure and the emitted light is analysed by a spectroscope, the spectrum consists of a large number of lines which are grouped into different series. The complete spectrum is known as hydrogen spectrum.
On the basis of experimental observations, Johannes Rydberg noted that all series of lines in the hydrogen spectrum could be described by the following expression:

![]()
Rydberg in 1890, and has given a simple theoretical equation for the calculation of wavelengths and wave numbers of the spectral lines in different series of hydrogen spectrum. The equation is known as Rydberg formula (or equation).
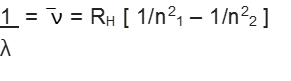
This relation is valid for hydrogen atom only. For other species,
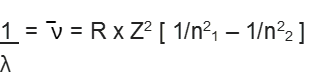
where Z is the atomic number of the species.
Here RH = constant, called Rydberg constant for hydrogen and n1 , n2 are integers (n2 > n1)
For any particular series, the value of n1 is constant while that of n2 changes. For example,
For Lyman series, n1= 1, n2= 2, 3, 4, 5………..
For Balmer series, n1 = 2, n2 = 3, 4, 5, 6………..
For Paschen series, n1= 3, n2 = 4, 5, 6, 7………..
For Brackett series,n1 = 4, n2 = 5, 6, 7, 8………..
For Pjund series, n1 =5, n2 = 6, 7, 8, 9………..
Thus, by substituting the values of n1 and n2 in the above equation, wavelengths and wave number of different spectral lines can be calculated. When n1 = 2, the expression given above is called Balmer’s formula.

 Ritan Sheth
Ritan Sheth
