- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
HYDROGEN BONDING
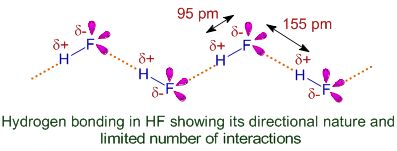
When highly electronegative elements like nitrogen, oxygen, flourine are attached to hydrogen to form covalent bond, the electrons of the covalent bond are shifted towards the more electronegative atom. Thus, partial positive charge develops on hydrogen atom which forms a bond with the other electronegative atom. This bond is known as hydrogen bond and it is weaker than the covalent bond. For example, in HF molecule, hydrogen bond exists between hydrogen atom of one molecule and fluorine atom of another molecule.
It can be depicted as
• Types of H-Bonds
(i) Intermolecular hydrogen bond (ii) Intramolecular hydrogen bond.
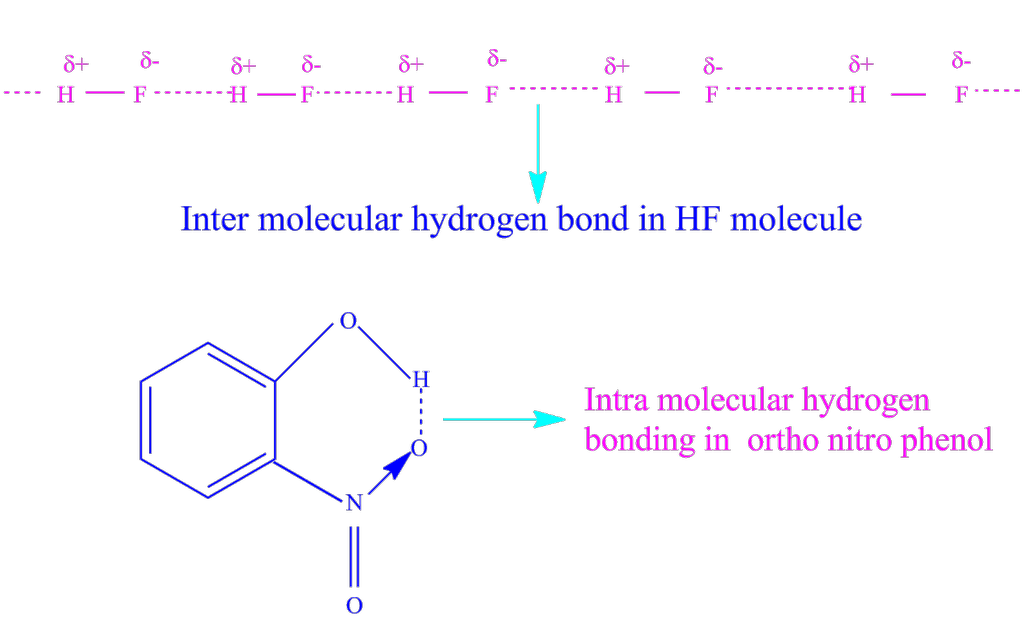
(i) Intermolecular hydrogen bond: It is formed between two different molecules of the same or different compounds. For Example, in HF molecules, water molecules etc.
(ii) Intramolecular hydrogen bond: In this type, hydrogen atom is in between the two highly electronegative F, N, O atoms present within the same molecule. For example, in o-nitrophenol, the hydrogen is in between the two oxygen atoms.

 Ritan Sheth
Ritan Sheth
