- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
STOICHIOMETRY AND STOICHIOMETRIC CALCULATIONS
The word ‘stoichiometry’ is derived from two Greek words—Stoicheion (meaning element) and matron (meaning measure). Stoichiometry, thus deals with the calculation of masses (sometimes volume also) of the reactants and the products involved in a chemical reaction. Let us consider the combustion of methane. A balanced equation for this reaction is as given below:
![]()
Hear, methane and dioxygen are called reactants and carbon dioxide and water are called products.

From these relationship the given data can be interconverted as follows
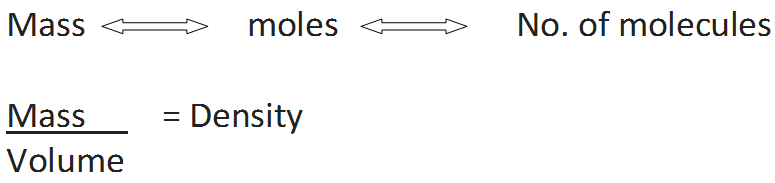
• Limiting Reactant/Reagent
Sometimes, in alchemical equation, the reactants present are not the amount as required according to the balanced equation. The amount of products formed then depends upon the reactant which has reacted completely. This reactant which reacts completely in the reaction is called the limiting reactant or limiting reagent. The reactant which is not consumed completely in the reaction is called excess reactant.
• Reactions in Solutions
When the reactions are carried out in solutions, the amount of substance present in its given volume can be expressed in any of the following ways:
1. Mass percent or weight percent (w/w%)
2. Mole fraction
3. Molarity
4. Molality
1. Mass percent: It is obtained by using the following relation:

2. Mole fraction: It is the ratio of number of moles of a particular component to the total number of moles of the solution. For a solution containing n2 moles of the solute dissolved in n1 moles of the solvent,


The sum of the mole fraction of the components is equal to 1
![]()
3. Molarity: It is defined as the number of moles of solute in 1 litre of the solution.
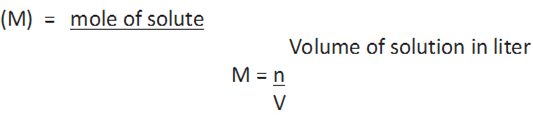
4. Molality: It is defined as the number of moles of solute present in 1 kg of solvent. It is denoted by m.

• All substances contain matter which can exist in three states — solid, liquid or gas.
• Matter can also be classified into elements, compounds and mixtures.
• Element: An element contains particles of only one type which may be atoms or molecules.
• Compounds are formed when atoms of two or more elements combine in a fixed ratio to each other.
• Mixtures: Many of the substances present around us are mixtures.
• Scientific notation: The measurement of quantities in chemistry are spread over a wide range of 10-31to 1023. Hence, a convenient system of expressing the number in scientific notation is used.
• Scientific figures: The uncertainty is taken care of by specifying the number of significant figures in which the observations are reported.
• Dimensional analysis: It helps to express the measured quantities in different systems of units.
• Laws of Chemical Combinations are:
(i) Law of Conservation of Mass
(ii) Law of Definite Proportions
(iii) Law of Multiple Proportions
(iv) Gay Lussac’s Law of Gaseous Volumes
(v) Avogadro’s Law.
• Atomic mass: The atomic mass of an element is expressed relative to 12C isotope of carbon which has an exact value of 12u.
• Average atomic mass: Obtained by taking into account the natural abundance of different isotopes of that element.
• Molecular mass: The molecular mass of a molecule is obtained by taking sum of atomic masses of different atoms present in a molecule.
• Avogadro number: The number of atoms, molecules or any other particles present in a given system are expressed in terms of Avogadro constant.
= 6.022 x 1023
• Balanced chemical equation: A balanced equation has the same number of atoms of each element on both sides of the equation.
• Stoichiometry: The quantitative study of the reactants required or the products formed is called stoichiometry. Using stoichiometric calculations, the amounts of one or more reactants required to produce a particular amount of product can be determined and vice-versa.

 Ritan Sheth
Ritan Sheth
