- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
NOMENCLATURE OF ORGANIC COMPOUNDS
An organic compound has two types of names – Common name and IUPAC name. The common name is based on the source or some properties. For e.g. citric acid is named so because it is found in citrus fruits and the acid found in red ant is named formic acid since the Latin word for ant is formica.
IUPAC Nomenclature of organic compounds
A systematic name of an organic compound is generally derived by identifying the parent hydrocarbon and the functional group(s) attached to it. This name is called IUPAC name. It contains two parts – word root and suffix or prefix. The word root indicates the number of carbon atoms in the compound. The word roots for compounds containing 1 -12 carbon atoms are as follows:
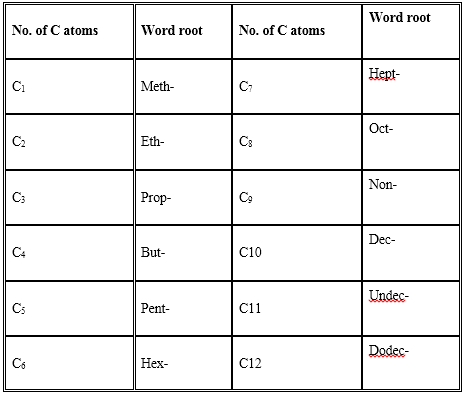
There are two types of suffixes – primary suffix and secondary suffix. Primary suffix indicates saturation or unsaturation [for alkane the primary suffix is –ane, alkene –ene and for alkyne –yne]. Secondary suffix indicates the type of functional group. Some functional groups are also indicated as prefixes.
Nomenclature of branched chain alkanes:
A branch (side chain or substituent) is obtained by removing a hydrogen atom from an alkane. The resulting group is called an alkyl group [alkane – H = alkyl (i.e. word root + yl)]. The names of some common branches are as follows:
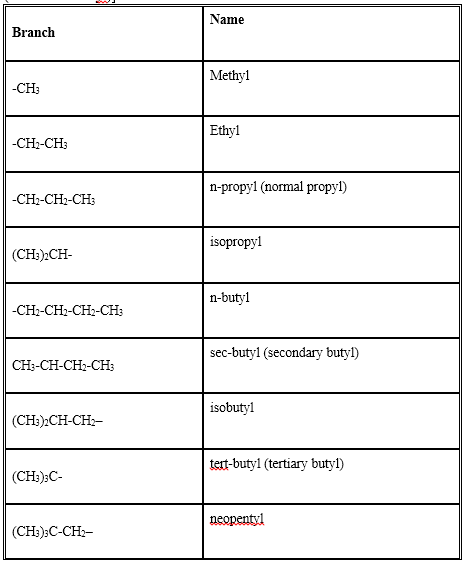
Rules for naming branched chain alkanes:
IUPAC recommenced the following rules for naming a branched chain alkane.
- Select the longest continuous chain of carbon atoms. This chain is called parent chain or root chain. If there is more than one such chain, the chain that contains maximum number of branches is selected as the parent chain. Also identify all the branches or substituents.
- Number the carbon atoms of the parent chain in such a way that the branched carbon atoms get the lowest possible numbers.
- The names of alkyl groups attached as branches are then prefixed to the name of the parent alkane and position of the substituents is indicated by the appropriate numbers.
- If different alkyl groups are present, they are listed in alphabetical order. In alphabetical order, the prefixes iso- and neo- are considered to be the part of the fundamental name of alkyl group. The prefixes sec- and tert- are not considered to be the part of the fundamental name.
- If two or more identical substituent groups are present then their numbers are indicated by prefixes like di (for 2), tri (for 3), tetra (for 4), penta (for 5) etc and the numbers are separated by commas. The number and word are separated by a hyphen. (The IUPAC name is written as a single word).
For example:
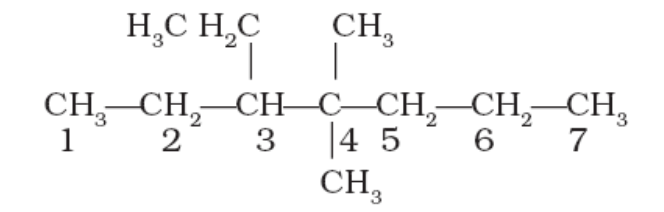
3-Ethyl-4,4-dimethylheptane
6. If the two substituents are found in equivalent positions, the lower number is given to the one coming first in the alphabetical listing. For example:
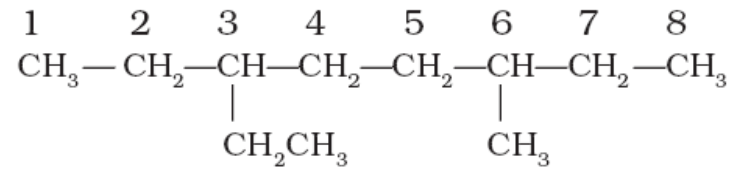
The above compound is 3-ethyl-6-methyloctane and not 6-ethyl-3-methyloctane.
7. While naming the branched alkyl groups, the carbon atom of the branch that attaches to the root alkane is numbered 1.
For example:
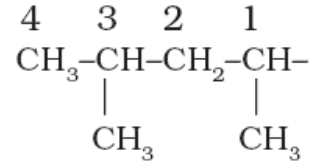
1,3-dimethyl butyl-
IUPAC nomenclature of compounds containing functional groups
For naming organic compounds containing functional group, the following rules are used:
- Select the longest continuous chain containing the functional group.
- Number the carbon atoms in such a way that the carbon to which the functional group is attached should get the lowest possible number. In the case of functional groups containing carbon atom like –CHO, -CN, – COOH, -CONH2, -COX. -COOR etc. the numbering should start from the carbon atom of the functional group. (i.e. carbon atom of these groups should be numbered as 1). (But for ketones, the functional group –CO- should get the lowest possible number).
- The name of the functional group is indicated by the following suffix or prefix.
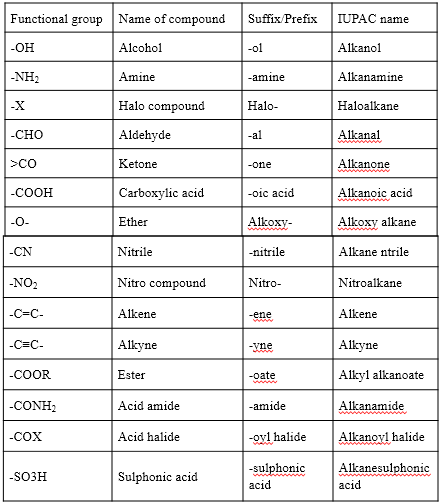
In the case of suffixes, the ending –e of the corresponding alkane is replaced. E.g. IUPAC name of the alcohol CH3-OH is methanol (methane + ol). But for nitriles, the –e of the corresponding alkane is retained.
E.g. IUPAC name of CH3-CH2-CN is propanenitrile.
In the case of alkenes and alkynes, the suffix –ane of the alkane is replaced by –ene and –yne respectively. (i.e. word root + ene or yne). For naming alkenes or alkynes, the numbering is done in such a way that the double or triple bond should get the lowest possible number.
Some examples are:
Nomenclature of organic compounds containing more than one functional groups (Poly functional compounds)
Here one of the functional groups is chosen as the principal functional group and the compound is named on that basis. The remaining functional groups (called subordinate functional groups) are named as substituents using the appropriate prefixes. The choice of principal functional group is made on the basis of order of preference. The order of decreasing priority for some functional groups is:
-COOH, –SO3H, -COOR (R=alkyl group), -COCl, -CONH2, -CN,-CHO, >CO, -OH, -NH2, >C=C<, -C≡C-
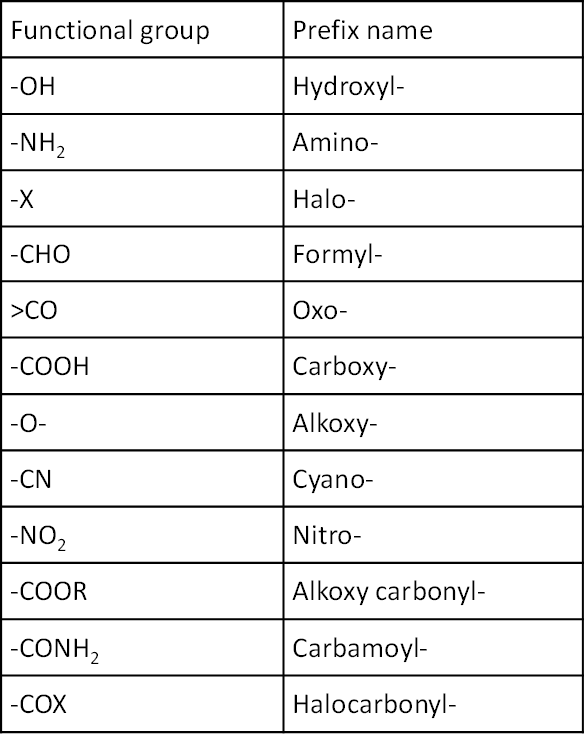
The groups like alkyl (–R), phenyl (C6H5-), halogens (F, Cl, Br, I), nitro (–NO2), alkoxy (–OR) etc. are always prefix substituents.
For example if a compound contains both alcoholic and aldehydic groups, it is named as hydroxyalkanal, since here aldehydic group is the principal functional group and –OH group is the subordinate functional group. The prefix names of some functional groups are as follows:
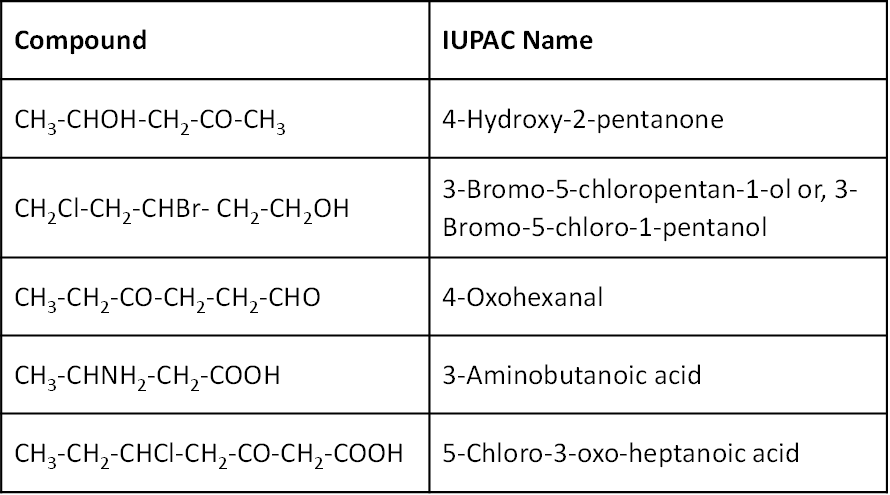
While numbering the carbon chain, the principal functional group should get the lowest possible number. Some examples are
If a compound contains more than one same functional group, their number is indicated by adding the numeral prefixes di, tri, etc. before the suffix. In such cases the full name of the parent alkane is written before the suffix. However, the ending – ne of the parent alkane is dropped in the case of compounds having more than one double or triple bonds.
When both double and triple bonds are present, the double bonds are given the lowest numbers. Here first give the suffix of the double bond (-en) and then that of the triple bond (-yne) [the ending –e of the suffix –ene is avoided].
Examples:
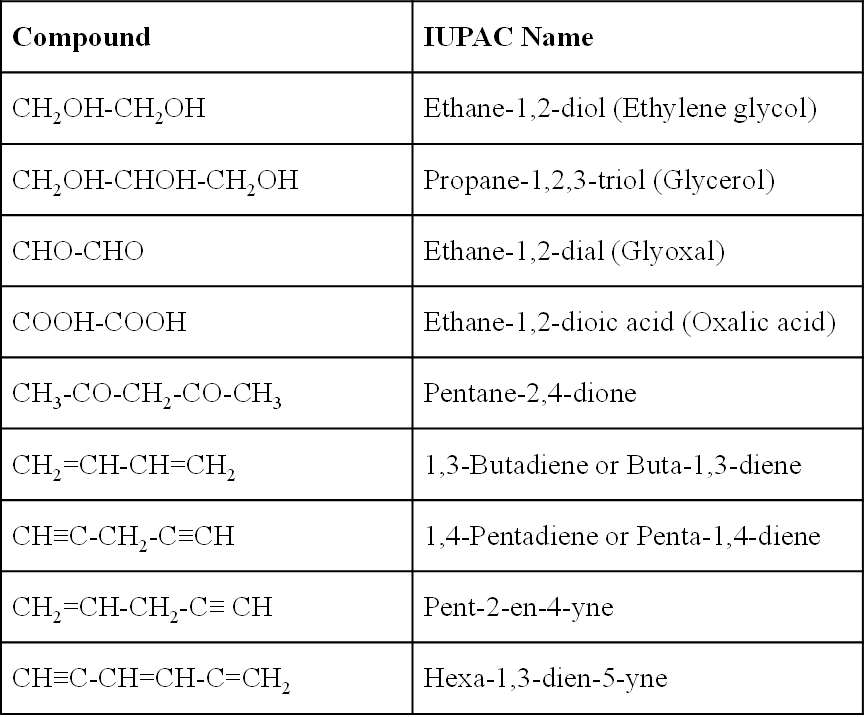
(The names given in the brackette are the common names)
Nomenclature of Substituted Benzene Compounds
For IUPAC nomenclature of substituted benzene compounds, the substituent is placed as prefix to the word benzene. But common names of some compounds are accepted by IUPAC.
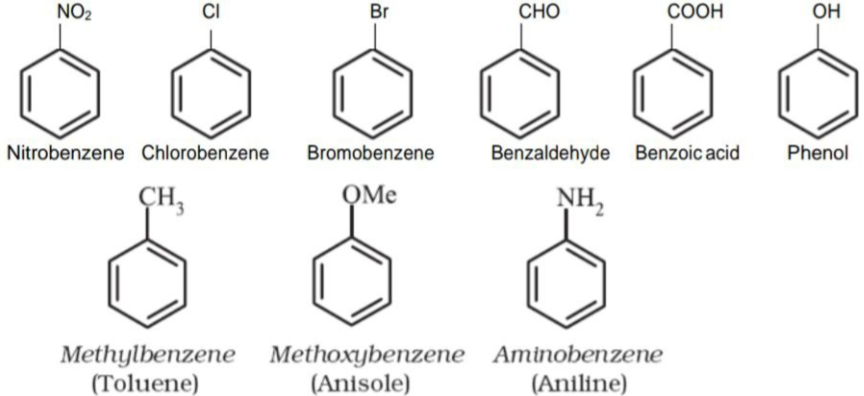
Nomenclatrue of di or polysubstituted benzene
If benzene ring is disubstituted, the position of substituents is indicated by numbering the carbon atoms of the ring such that the substituents get the lowest possible numbers.
Example – Dibromobenzene
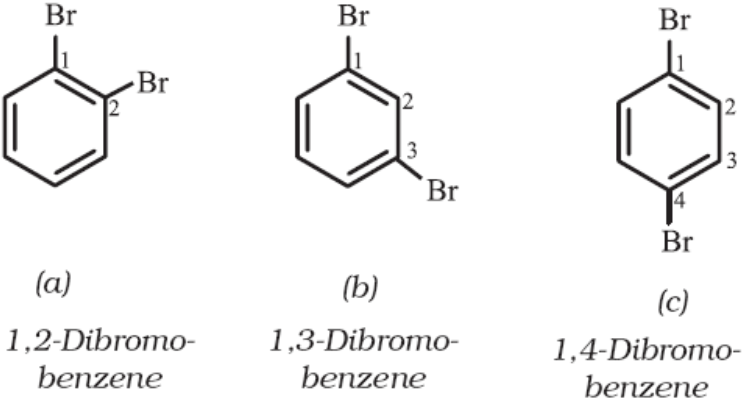
In the common system of nomenclature the terms ortho (o), meta (m) and para (p) are used as prefixes to indicate the relative positions 1,2- 1,3- and 1,4- respectively. So 1,2-dibromobenzene is named as ortho (or just o-) dibromobenzene, 1,3-dibromobenzene as meta (or just m-) dibromobenzene and 1,4-dibromobenzene as para (or just p-)-dibromobenzene.
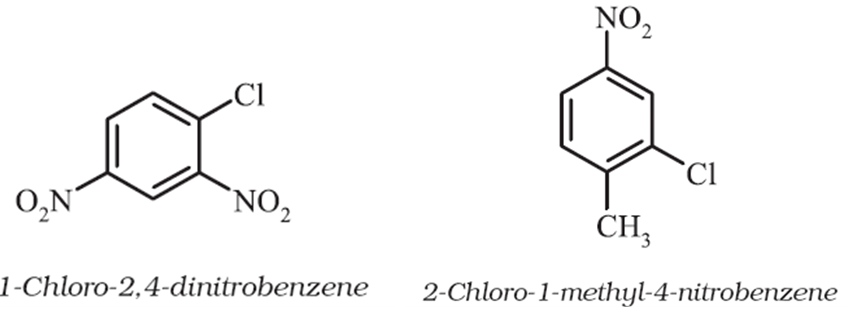
For tri – or higher substituted benzene derivatives, these prefixes cannot be used and the compounds are named by identifying substituent positions on the ring by following the lowest locant rule. In some cases, common name of benzene derivatives is taken as the base compound. Substituent of the base compound is assigned number1 and then the direction of numbering is chosen such that the next substituent gets the lowest number. The substituents are named in alphabetical order.
Some examples are:
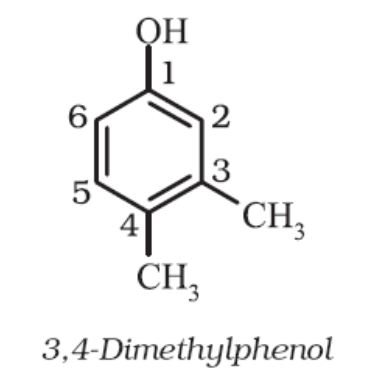
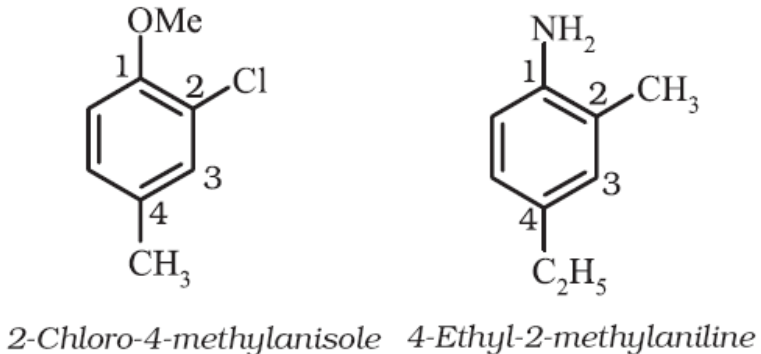
When a benzene ring is attached to an alkane with a functional group, it is considered as substituent, instead of a parent. The name for benzene as substituent is phenyl (C6H5-, also abbreviated as Ph).
Example:
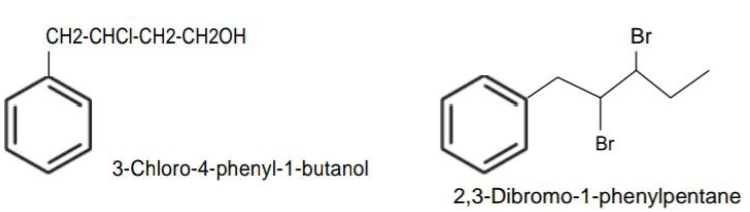

 Ritan Sheth
Ritan Sheth
