- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
QUANTUM MECHANICAL MODEL OF ATOM
Quantum mechanics: Quantum mechanics is a theoretical science that deals with the study of the motions of the microscopic objects that have both observable wave like and particle like properties.
Important Features of Quantum Mechanical Model of Atom
(i) The energy of electrons in atom is quantized i.e., can only have certain values.
(ii) The existence of quantized electronic energy level is a direct result of the wave like properties of electrons.
(iii) Both the exact position and exact velocity of an electron in an atom cannot be determined simultaneously.
(iv) An atomic orbital has wave function φ. There are many orbitals in an atom. Electron occupy an atomic orbital which has definite energy. An orbital cannot have more than two electrons. The orbitals are filled in increasing order of energy. All the information about the electron in an atom is stored in orbital wave function φ.
(v) The probability of finding electron at a point within an atom is proportional to square of orbital wave function i.e., |φ2|at that point. It is known as probability density and is always positive.
From the value of φ2 at different points within atom, it is possible to predict the region around the nucleus where electron most probably will be found.
• Quantum Numbers
Atomic orbitals can be specified by giving their corresponding energies and angular momentums which are quantized (i.e., they have specific values). The quantized values can be expressed in terms of quantum number. These are used to get complete information about electron i.e., its location, energy, spin etc.
Principal Quantum Number (n)
It is the most important quantum number since it tells the principal energy level or shell to which the electron belongs. It is denoted by the letter V and can have any integral value except zero, i.e., n = 1, 2, 3, 4……….. etc.
The various principal energy shells are also designated by the letters, K, L, M, N, O, P ….. etc. Starting from the nucleus.
The principal quantum number gives us the following information:
(i) It gives the average distance of the electron from the nucleus.
(ii) It completely determines the energy of the electron in hydrogen atom and hydrogen like particles.
(iii) The maximum number of electrons present in any principal shell is given by 2n2 where n is the number of the principal shell.
Azimuthal or Subsidiary or Orbital Angular Quantum Number (l)
It is found that the spectra of the elements contain not only the main lines but there are many fine lines also present. This number helps to explain the fine lines of the spectrum.
The azimuthal quantum number gives the following information:
(i) The number of subshells present in the main shell.
(ii) The angular momentum of the electron present in any subshell.
(iii) The relative energies of various subshells.
(iv) The shapes of the various subshells present within the same principal shell.
This quantum number is denoted by the letter T. For a given value of n, it can have any value ranging from 0 to n – 1. For example,
For the 1st shell (k), n = 1, l can have only one value i.e., l = 0 For n = 2, the possible value of l can be 0 and 1.
Subshells corresponding to different values of l are represented by the following symbols:
value of l 0 1 2 3 4 5 ……………..
Notation for subshell s p d f g h ………………..
Magnetic Orbital Quantum Number (m or m1)
The magnetic orbital quantum number determines the number of preferred orientations of the electrons present in a subshell. Since each orientation corresponds to an orbital, therefore, the magnetic orbital quantum number determines the number of orbitals present in any subshell.
The magnetic quantum number is denoted by letter m or ml and for a given value of l, it can have all the values ranging from – l to + l including zero.
Thus, for energy value of l, m has 2l + 1 values.
For example,
For l = 0 (s-subshell), ml can have only one value i.e., m1 = 0.
This means that s-subshell has only one orientation in space. In other words, s-subshell has only one orbital called s-orbital.
Spin Quantum Number (S or ms)
This quantum number helps to explain the magnetic properties of the substances. A spinning electron behaves like a micro magnet with a definite magnetic moment. If an orbital contains two electrons, the two magnetic moments oppose and cancel each other.
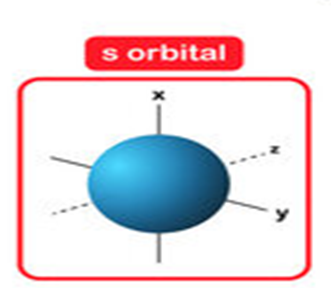
• Shapes of s-orbitals
s-orbital is present in the s-subshell. For this subshell, l = 0 and ml = 0. Thus, s-orbital with only one orientation has a spherical shape with uniform electron density along all the three axes.
The probability of Is electron is found to be maximum near the nucleus and decreases with the increase in the distance from the nucleus. In 2s electron, the probability is also maximum near the nucleus and decreases to zero probability. The spherical empty shell for 2s electron is called nodal surface or simply node.
![]()
• Shapes of p-orbitals
p-orbitals are present in the p-subshell for which l = 1 and m1 can have three possible orientations – 1, 0, + 1.
Thus, there are three orbitals in the p-subshell which are designated as px, py and pz orbitals depending upon the axis along which they are directed. The general shape of a p-orbital is dumb-bell consisting of two portions known as lobes. Moreover, there is a plane passing through the nucleus along which finding of the electron density is almost nil. This is known as nodal plane as shown in the fig.
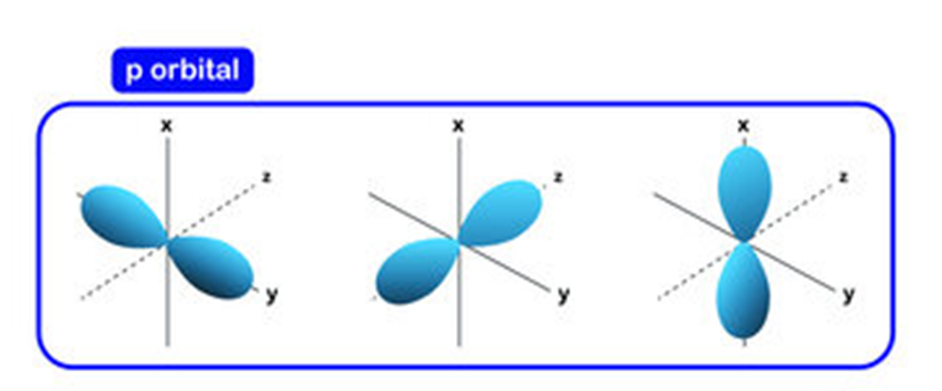
From the dumb-bell pictures, it is quite obvious that unlike s-orbital, a p-orbital is directional in nature and hence it influences the shapes of the molecules in the formation of which it participates.
• Shapes of d-orbitals
d-orbitals are present in d-subshell for which l = 2 and m[ = -2, -1, 0, +1 and +2. This means that there are five orientations leading to five different orbitals.
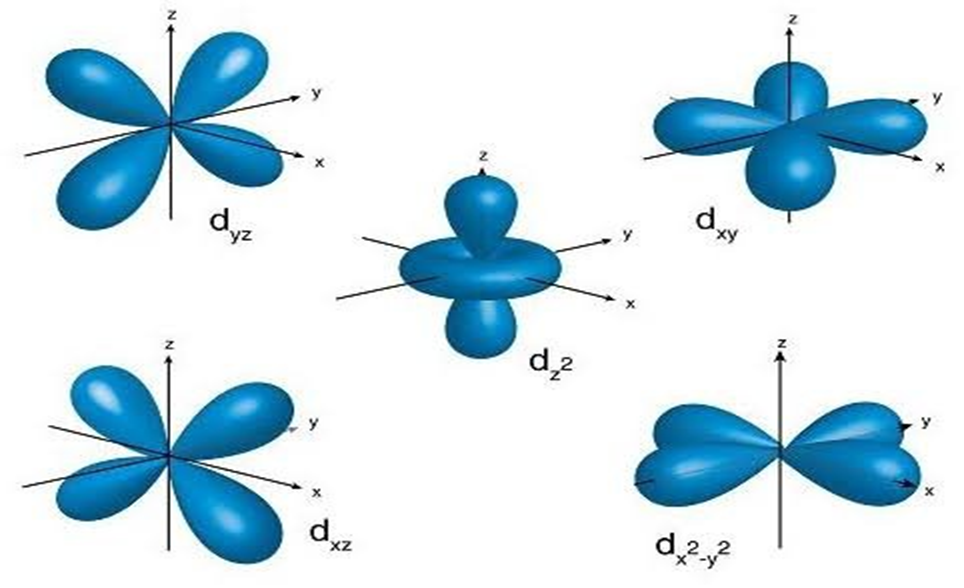
These five orientation are designated as dzy , dyz , dzx , dx2-y2 , dz2 . However they have the same energy are in degeneracy state and are known as degenerated orbitals. The first three orbitals have clover leaf shape and lie in different planes which are xy,yz,and zx planes respectively. The dx2-y2 orbital is also clover leaf shaped but its lobes are directed along the X and Y axis.
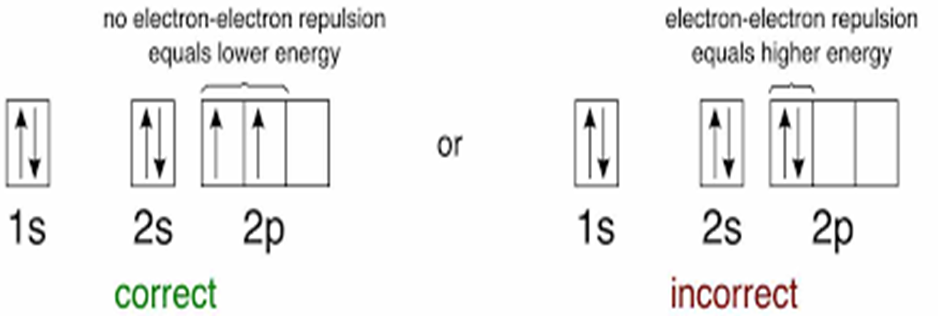
• Aufbau Principle
The principle states: In the ground state of the atoms, the orbitals are filled in order of their increasing energies.
In other words, electrons first occupy the lowest energy orbital available to them and enter into higher energy orbitals only after the lower energy orbitals are filled.
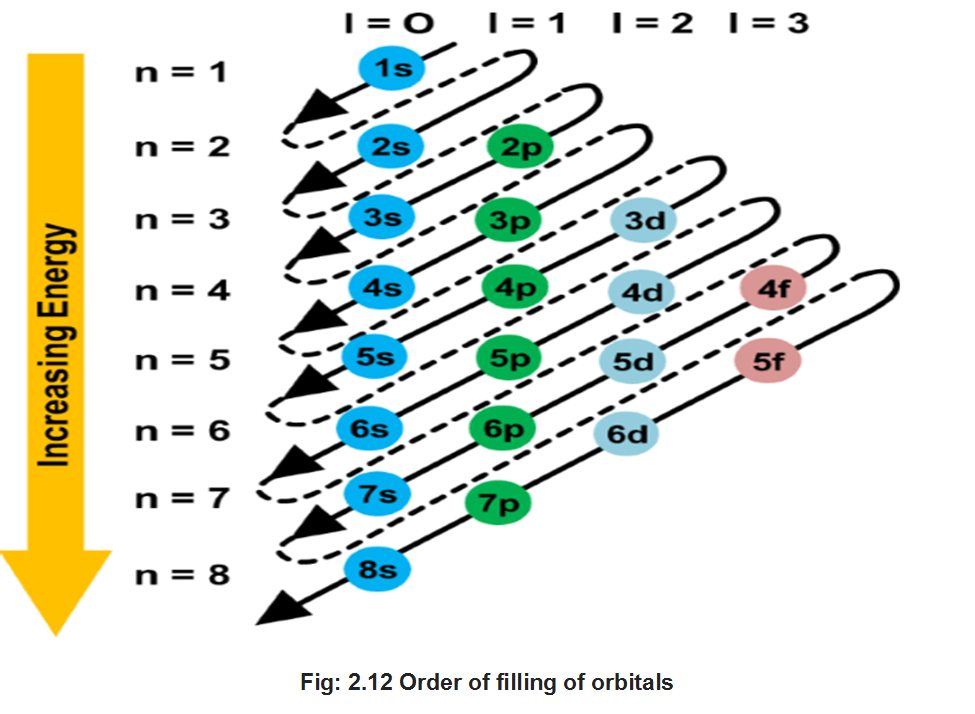
The order in which the energies of the orbitals increase and hence the order in which the orbitals are filled is as follows:
Is, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, id, 5p, 6s, if, 3d, 6p, 7s, 5f 6d, 7p
The order may be remembered by using the method given in fig. 2.11.
• Pauli Exclusion Principle
According to this principle, no two electrons in an atom can have the same set of four quantum numbers.
Pauli exclusion principle can also be stated as: Only two electrons may exist in the same orbital and these electrons must have opposite spins.
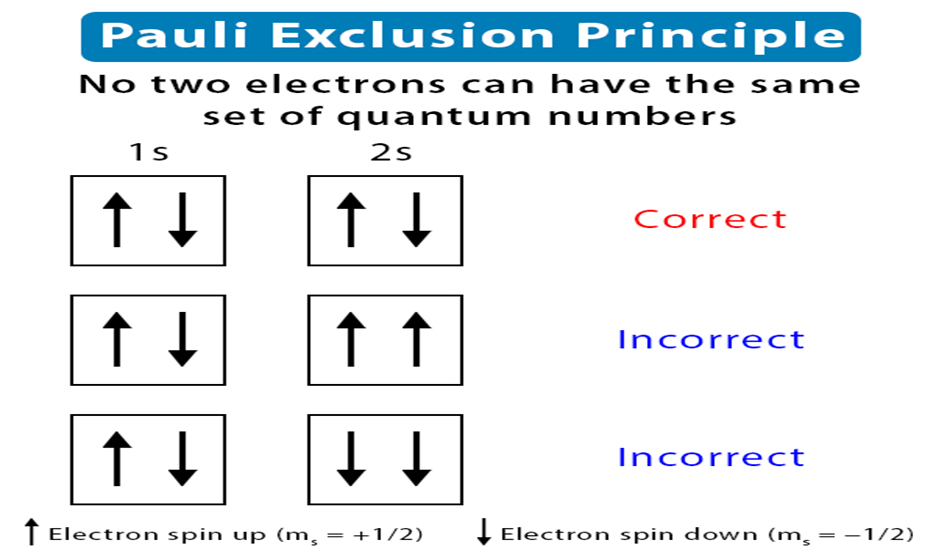
• Hund’s Rule of Maximum Multiplicity
It states that: pairing of electrons in the orbitals belonging to the same subshell (p, d or f) does not take place until each orbital belonging to that subshell has got one electron each i.e., it is singly occupied.
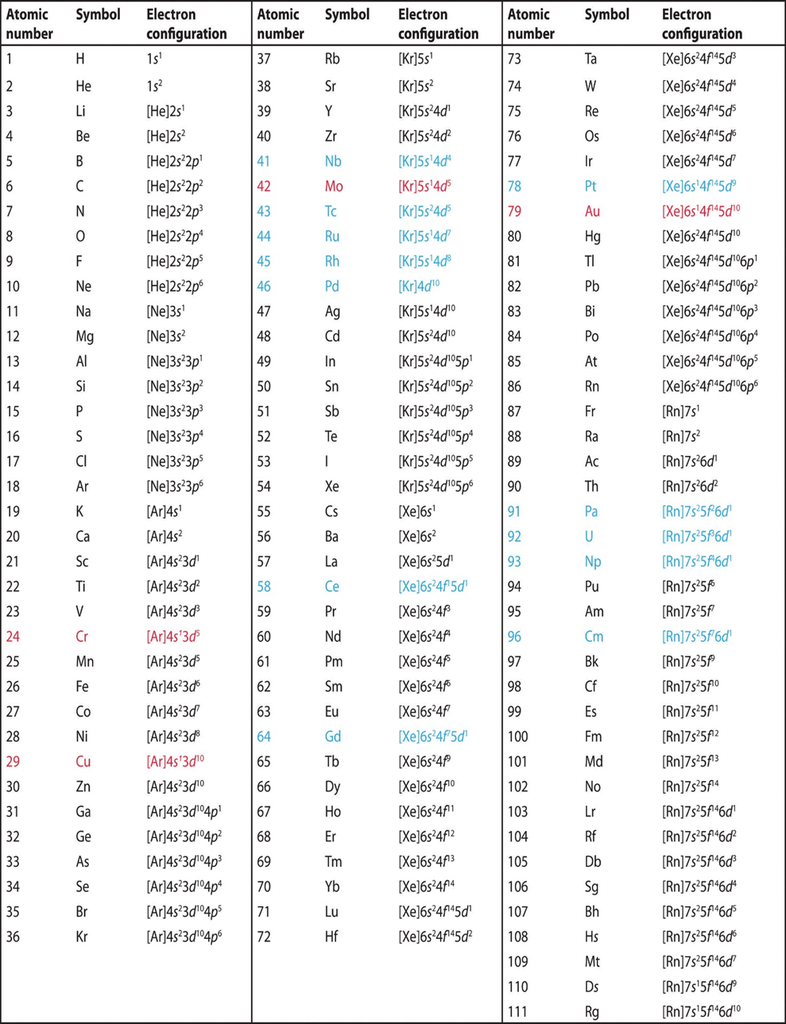
• Electronic Configuration of Atoms
The distribution of electrons into orbitals of an atom is called its electronic configuration. The electronic configuration of different atoms can be represented in two ways.
For example:
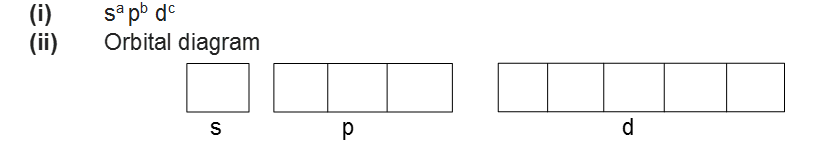
For example: the electronic configuration of hydrogen is

• Causes of Stability of Completely Filled and Half Filled Subshells
The completely filled and half filled subshells are stable due to the following reasons:
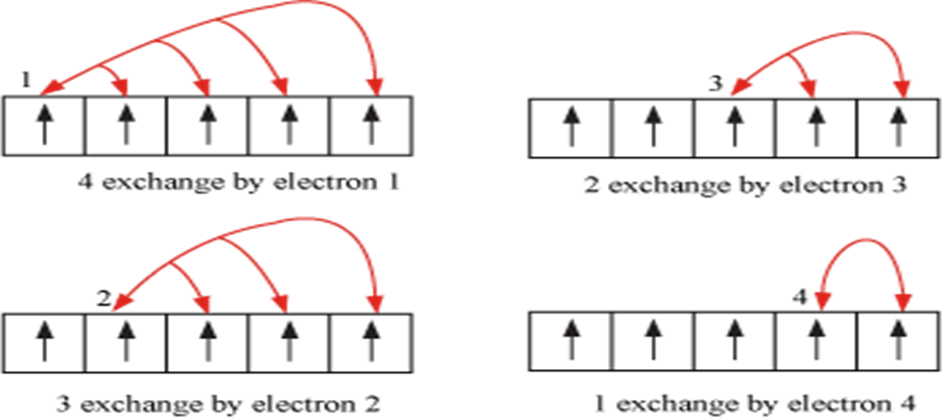
1. Symmetrical distribution of electrons: The completely filled or half filled subshells have symmetrical distribution of electrons in them and are therefore more stable.
2. The stabilizing effect arises whenever two or more electrons with same spin are present in the degenerate orbitals of a subshell. These electrons tend to exchange their positions
and the energy released due to their exchange is called exchange energy. The number of exchanges that can takes place is maximum when the subshell is either half filled or completely filled.
-As a result the exchange energy is maximum and so is the stability.

 Ritan Sheth
Ritan Sheth
