- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
GIBBS ENERGY CHANGE AND EQUILIBRIUM
At equilibrium, i.e., ΔG = 0, the process is neither spontaneous nor non-spontaneous because it is balanced between spontaneous and non-spontaneous behavior. (+ΔH)
So,
ΔG = ΔH – T ΔS = 0
Hence,
ΔH = TΔS or T = ΔH / ΔS
T is the temperature at which the transition from spontaneous to non-spontaneous behaviour happens. T is calculated on the assumption that ΔH and ΔS are temperature independent. In reality, ΔH and ΔS change with temperature. However, for modest temperature changes, the variance in them will not add considerable mistakes.
ΔG and Equilibrium constant
All of the substances (reactants and products) in a chemical reaction may not be in their normal forms. As a result of the connection, the change in Gibbs energy of a reaction is related to the change in standard Gibbs energy.
ΔG = ΔG° + RT ln Q
where:
ΔG° is the standard Gibbs energy change (change in Gibbs energy when all the substances are in their standard state).
Q is the reaction quotient.
The expression of the reaction quotient is similar to that of the equilibrium constant, but there is one single difference between them, i.e., Equilibrium concentrations or partial pressures of products and reactants are included in the equilibrium constant. Whereas Q is expressed in terms of reactant beginning concentration partial pressures and product final concentrations or pressures.
For Example, consider the below example:
aA +bB ⇢ cC + dD
For the above reaction, the reaction Quotient is given by
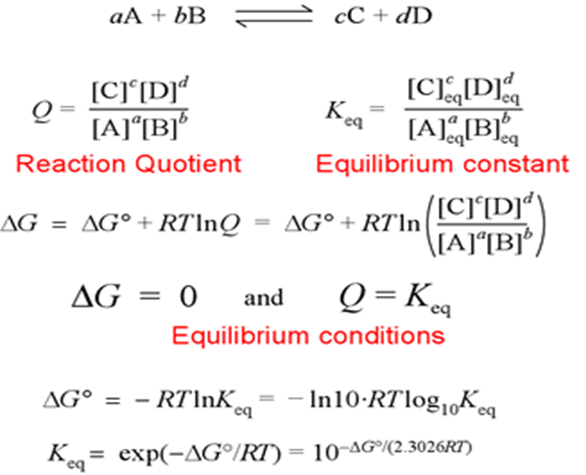
When the values of concentration or partial pressure are other than equilibrium values. When the reaction reaches equilibrium, the concentrations and partial pressure reach their equilibrium values and at this stage, Q = K. At equilibrium, ΔG = 0 and Q = K, then the standard Gibbs energy equation becomes,
0 = ΔG° + RT ln K
Hence,
ΔG° = -RT ln K = -2.303RT log10K
This equation gives the relationship between standard Gibbs energy change for the reaction and its equilibrium constant.

 Ritan Sheth
Ritan Sheth
