1. Occurrence of metals old
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chapter 6
General principles and processes of isolation of elements
Introduction :
The compound of a metal found in nature is called a mineral. The minerals from which metal can be economically and conveniently extracted are called ores. An ore is usually contaminated with earthy or undesired materials known as gangue. So all minerals are not ores but all ores are minerals. Ores may be classified mainly into following four classes.
(a) Native ores : They contain the metal in free state. Silver, gold, platinum etc, occur as native ores.
(b) Oxidised ores : These ores consist of oxides or oxysalts (e.g. carbonates, phosphates, sulphates and silicates ) of metals.
(c) Sulphurised ores : These ores consist of sulphides of metals like iron, lead, zinc, mercury etc.
(d) Halide ores : These ores consist of halides of metals.
Important ore :
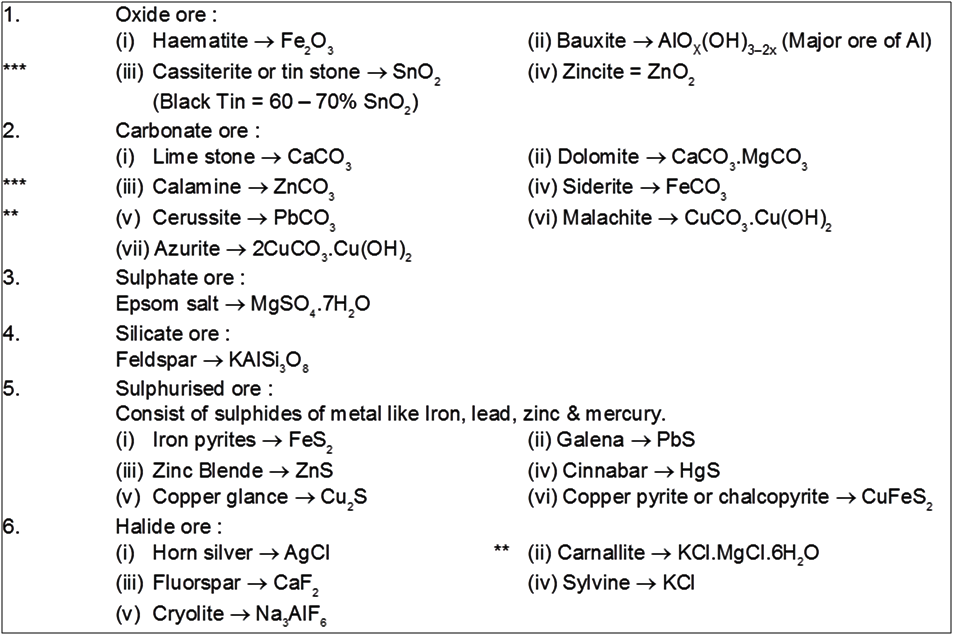
Note : Mg obtained from both sea water & earth crust.
Metallurgy :
The scientific and technological process used for the extraction/isolation of the metal from its ore is called as metallurgy.
The isolation and extraction of metals from their ores involve the following major steps:
(A) Crushing of the ore.
(B) Dressing or concentration of the ore.
(C) Isolation of the crude metal from its ore
(D) Purification or refining of the metal.
Chart1:
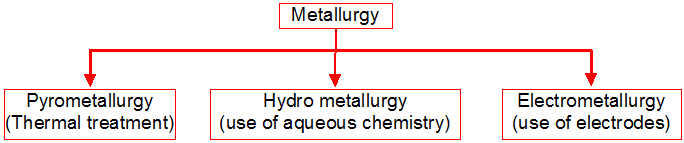
Chart2: Steps involved in metallurgy.
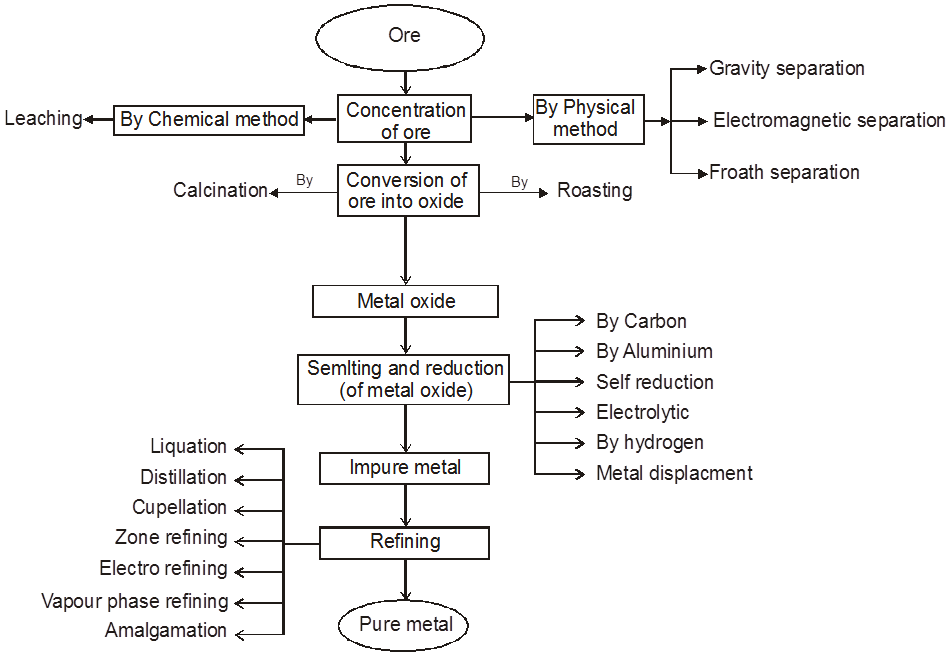
1. Physical Method :
(A) Crushing and Grinding : The ore is first crushed by jaw crushers and ground to a powder (pulverisation of the ore) in equipments like ball mills and stamp mills.
(B) Concentration : The removal of unwanted useless impurities from the ore is called dressing, concentration or benefaction of ore.
It involves several steps and selection of these steps depends upon the difference in physical properties of the compound of metal and that of gangue. Some of the important procedures are described below.
(i) Hydrolytic washing :
Gravity separation or "Levigation". Based on the difference in the densities of the gangue and ore particle.
Generally used for the concentration of oxide & native ore.
(ii) Electromagnetic sepration :
Based on difference in magnetic properties of mineral and gangue particle.
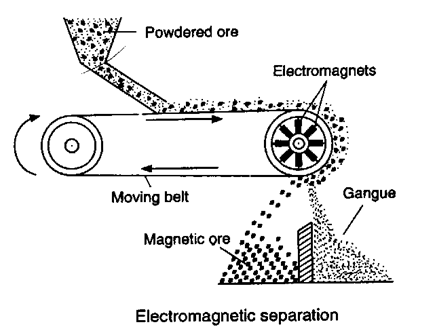
(a) Chromite ore [FeO.Cr2O3] is seprated from non magnetic silicious impurities.
(b) Cassiterite ore [SnO2] is seprated from magnetic wolframite [FeWO4 + MnWO4]
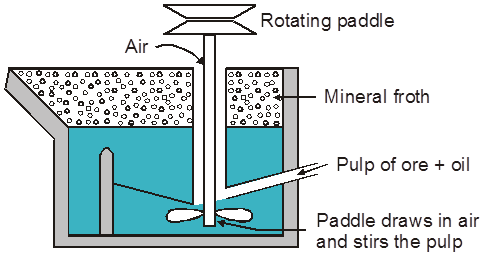
(iii) Froth floatation process :
Generally used for concentration of low grade sulphide ore PbS, ZnS, Cu2S, CuFeS2
Principle :
Based on fact that mineral & gangue particles have different wettability in water and oil (pine oil used)
Mineral particle ® are wetted by oil.
Gangue particle ® Wetted by water.
Reagents Used :
(i) Frothers :
These form stable froth which rises to the top of the flotation cell.
Oil like pine oil, comphor oil are used in small quantities.
Main Function of :
Frother ® Stick to ore & then take it to rise upto the top.
Stabilizer ® To stabilize the froth, froth stabilizer like [cresol & aniline] are added.
(ii) Collector :
K+ or Na+ ethyl xanthates![]() (R = alkyl,M+ = Na+ , K+) are used as collectors. Which collect or attract mineral partical and make them water repellant.
(R = alkyl,M+ = Na+ , K+) are used as collectors. Which collect or attract mineral partical and make them water repellant.
Main Function : Make the ore hydrophobic.
(iii) Activating & Depressing agents :
In PbS + ZnS + FeS2 mixture NaCN + Na2CO3 is used to depress the floation properties of ZnS & FeS2.
ZnS + CN– ¾® [Zn(CN)4]2–
FeS2 + CN– ¾® [Fe(CN)6]4–
CuSO4 is then added which is activating for ZnS as Cu forms more stable complexing with CN– than Zn2+
2. Chemical Method :
(4) Leaching :
Used when ore is soluble in some suitable solvent like ® acid, base & suitable chemical reagent.
Ex. (a) Leaching of alumina from bauxite.
(b) Extraction of Ag & Au from their ores in the complex form by treatment with NaCN & KCN.
(c) Treatment of low grade Cu ores with conc. H2SO4.
(C) Extraction of crude metal from concentrated ore :
The concentrated ore must be converted into a form which is suitable for reduction. Usually the sulphide ore is converted to oxide before reduction. Oxides are easier to reduce. Thus isolation of metals from concentrated ore involves two major steps as given below.
(i) Conversion to oxide
(ii) Reduction of the oxide to metal.
(i) Conversion to oxide :

Conversion of ore into oxide is carried out in two ways depending upon the nature of ore.
Calcination. It is a process of heating the concentrated ore strongly in a limited supply of air or in the absence of air. The process of calcination brings about the following changes :
(a) The carbonate ore gets decomposed to form the oxide of the metal, e.g.,
FeCO3 (siderite) ![]() FeO + CO2
FeO + CO2
PbCO3 (cerrussite) ![]() PbO + CO2
PbO + CO2
CaCO3 (calcite ore / lime stone) ![]() CaO + CO2
CaO + CO2
ZnCO3 (calamine) ![]() ZnO + CO2
ZnO + CO2
CuCO3.Cu(OH)2 (malachite) ![]() 2CuO + H2O + CO2
2CuO + H2O + CO2
MgCO3.CaCO3 (dolomite) ![]() MgO + CaO + 2CO2
MgO + CaO + 2CO2
(b) Water of crystallisation present in the hydrated oxide ore gets lost as moisture, e.g.,
2Fe2O3.3H2O (limonite) ![]() 2Fe2O3(s) + 3H2O(g)
2Fe2O3(s) + 3H2O(g) 
Al2O3. 2H2O (bauxite) ![]() Al2O3 (s) + 2H2O(g)
Al2O3 (s) + 2H2O(g) 
c) Organic matter, if present in the ore, gets expelled and the ore becomes porous. Volatile impurities are removed.
Roasting :
Generally used for sulphide ore.
Process : Concentrated ore is strongly heated in excess of air or O2 below its metling point.
(a) Roasting at moderate temperature :
2PbS + 3O2 ![]() 2PbO + 2SO2
2PbO + 2SO2
2ZnS + 2O2 ![]() 2ZnO + 2SO2
2ZnO + 2SO2
If temperature is low (500°C) & concentration of SO2 is high sulphate are produced.
PbS + 2O2 ![]() PbSO4
PbSO4
ZnS + 2O2 ![]() ZnSO4
ZnSO4
(b) Roasting at High temperature :
Self reduction / auto reduction / air reduction :
Sulphide ore of Cu, Pb, Hg & Sb when strongly heated in free supply of air, directly reduced to the metal this known as self reduction.
Cu2S (Copper glance) + O2 ¾® 2Cu + SO2
PbS (Gelena) + O2 ¾® Pb + SO2
HgS (Cinabar) + O2 ¾® Hg + SO2
Important Points
1.It remove impurities of As as As2O3, sulphur as SO2, P as P4O10 & Sb as Sb2O3
4M (M = As, Sb) + 3O2 ¾® 2M2O3↑
S + O2 ¾® SO2↑
P4 + 4O2 ¾® P4O10↑
2. Impurities of CuS & FeS in SnO2 converted to CuSO4 & FeSO4
CuS + 2O2 ![]() CuSO4
CuSO4
FeS + 2O2 ![]() FeSO4
FeSO4
Note : Calcination & Roasting carried out in a reverberatory furnace.
Smelting :
Slag formation : In many extraction processes, an oxide is added deliberately to combine with other impurities and form a stable molten phase immiscible with molten metal called a slag. The process is termed smelting.
The principle of slag formation is essentially the following :
Nonmetal oxide (acidic oxide) + Metal oxide (basic oxide) ¾® Fusible (easily melted) slag
Removal of unwanted basic and acidic oxides: For example, FeO is the impurity in extraction of Cu from copper pyrite.
2CuFeS2 + 4O2 ¾® Cu2S + 2FeO + 3SO2
Cu2S + FeO + SiO2 ¾® FeSiO3 (Fusible slag) + Cu2S (matte)
![]() (upper layer) (lower layer)
(upper layer) (lower layer)
Matte also contains a very small amount of iron(II) sulphide.
To remove unwanted acidic impurities like sand and P4O10, smelting is done in the presence of limestone.
CaCO3 ¾® CaO + CO2
CaO + SiO2 ¾® CaSiO3 (fusible slag)
6CaO + P4O10 ¾® 2Ca3(PO4)2 (fusible slag - Thomas slag)
Properties of a slag :
(i) Slag is a fusible mass.
(ii) It has low melting point.
(iii) It is lighter than and immiscible with the molten metal. It is due to these impurities that the slag floats as a separate layer on the molten metal and can thus be easily separated from the metal. The layer of the slag on the molten metal prevents the metal from being oxidised.
Type of flux : Fluxes are of two types viz., acidic flux and basic flux.
(a) Acidic flux : It is an acidic oxide (oxide of a non-metal) like SiO2, P2O5, B2O3 (from borax). It is used to remove the basic impurity like CaO, FeO, MgO etc. The acidic flux combines with the basic impurity and forms a slag.
(b) Basic flux : It is a basic oxide (i.e., oxide of a metal) like CaO (obtained from lime stone, CaCO3), MgO (from magnesite, MgCO3), haematite (Fe2O3) etc. It is used to remove the acidic impurity like SiO2, P2O5 etc. The basic flux combines with the acidic impurity and forms a slag.
Thus, slag can be defined as a fusible mass, which is obtained when a flux reacts with an infusible acidic or basic impurity present in the oxide ore.
Reduction of a Metal Oxide
(1) Reduction with Carbon :
PbO + C ¾® Pb + CO
2Fe2O3 + 3C ¾® 4Fe + 3CO2
*** ZnO + C ![]() Zn + CO (Extraction of Zn)
Zn + CO (Extraction of Zn)
*** SnO2 + 2C ![]() Sn + 2CO
Sn + 2CO
(2) Reduction with CO :
Fe2O3 + 3CO ¾® 2Fe + 3CO2
Fe3O4 + 4CO ¾® 3Fe + 4CO2
Reduction with C & CO is carried out in blast furnace.
(3) Redution with Al :
GoldSchmidt or Aluminothermic process :
Metallic oxide of Cr & Mn reduced by Al & this reaction is known as thermite reaction.
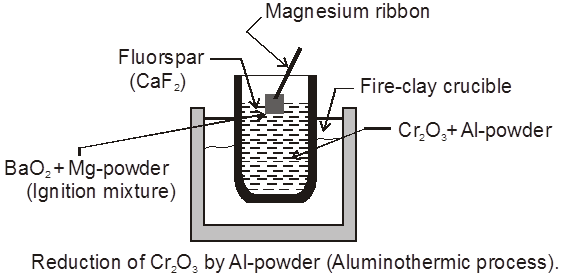
Mg + BaO2 ¾® BaO + MgO + Heat
(i) Cr2O3 + Al ¾® 2Cr(l) + Al2O3
*** (ii) 3Mn3O4 + 8Al ¾® 4Al2O3 + 9Mn
(iii) 2Al + Fe2O3 ¾®Al2O3 + 2Fe
(iv) B2O3 + 2Al ¾® 2B + Al2O3
(4) Redution by Mg & Na :
(i) TiCl4 + 2Mg ![]() Ti + 2MgCl2
Ti + 2MgCl2
(ii) TiCl4 + 4Na ![]() Ti + 4NaCl
Ti + 4NaCl
(5) Self reduction or auto reduction or air reduction :
Sulphide ore of some of the metal like Hg, Cu, Pb & Sb, when heated in air some part of these ore change into oxide or sulphate, thenthat part react with remaing part of sulphide ore to give its metal & sulphur dioxide.
This process is known as self reduction.
Ex. (i)(a) 2PbS + 3O2 ¾®2PbO + 2SO2
2PbO + PbS ¾®3Pb + SO2
(b) PbS + 2O2 ¾®PbSO4
PbSO4 + PbS ¾®2Pb + 2SO2
(ii) 2HgS + 3O2 ¾®2HgO + 2SO2
2HgO + HgS ¾®3Hg + SO2
(6) Electrolytic Reduction :
Electrolytic reduction is an expensive method than chemical method, that's why generally we do not use this method.
But when very high pure metal is required then we use this method.
This method is also used for highly reactive metal.
(i) Electrolyric reduction in aqueous solution :
This method is used when product does not react with water.
Electrolytic reduction of Cu & Zn from their sulphates.
(ii) In other solvents : Flourine react with water so it is produced by electrolysis of KHF2 dissolved in anhydrous HF.
(iii) In fused metal : When produced metal react with water, then metal is extracted from fused melt of their ionic salt.
Eg. (i) Al is extracted from electrolysis of fused mixture of Al2O3 and cryolite (Na3AlF6).
(ii) Extraction of Na by electrolysis of fused NaCl.
Note : In this electrolysis CaCl2 is added as impurity to lower the melting point from 800°C to 500°C.
(i) Hydro Metallurgy :
When metal can be extracted using solution without any heating or without any electrolysis then operation is known as hydrometallurgy.
Basic Step
(i) Dissolution of the valuable metal in aqueous solution.
(ii) Purification of leach solution.
(iii) Recovery of metal from purified solution.
Ex. Extraction of Ag & Au :
Metallic Ag dissolved in NaCN from ore of Ag & then precipitated with the help of Zn.
AgS(s) + 4CN¯ ¾® ![]() + S2–
+ S2–
2[Ag(CN)2]¯(aq) + Zn(s) ¾® [Zn(CN)4]2–(aq) + 2Ag(s)
(ii) Pyrometallurgy :
If furnace are used and ore are heated to extract metal then it is called pyrometallurgy.
Electrochemical principles of metallurgy :
Electrolytic reduction can be regarded as a technique for driving a reduction by coupling it through electrodes and external circuit to a reactive or a physical process with a more negative DG. The free energy available from the external source can be assessed from the potential it produces across the electrodes using the thermodynamic relation :
DG = –nFE ..........(i)
where n is the number of electrons transferred, F is Faraday’s constant (F = 96.5 kJ/mol) and Eº is electrode potential of the redox coupled formed in the system.
Hence, the total Gibb’s energy of the coupled internal and external process is
DG + DG (external) = DG – nFEext
If the potential difference of the external source exceeds
Eext = –![]()
the reduction is thermodynamically feasible; thus, the overall process occurs with a decrease in free energy.
More reactive metals have large negative values of the electrode potential. So their reduction is difficult. If the difference of two E0 values corresponds to a positive E0 and consequently negative D G0 in equation (i), then the less reactive metal will come out of the solution and the more reactive metal will go to the solution, e.g.,
Cu2+ (aq) + Fe(s) —® Cu(s) + Fe2+(aq)
In simple electrolysis, the Mn+ ions are discharged at negative electrodes (cathodes )and deposited there. Precautions are taken considering the reactivity of the metal produced and suitable materials are used as electrodes. Sometimes a flux is added for making the molten mass more conducting.
Hydrometallurgy : The processing of ores and minerals as well as metals and their compounds at relatively low, often ambient temperatures employing aqueous solution is known as hydrometallurgy. Occasionally, organic reagents are also used. This method of extraction is generally used for low grade ores. Copper is extracted by hydrometallurgy from low grade ore it is leached out using acid and bacteria. The solution containing Cu2+ is treated with scrap iron or H2.
CuSO4 + Fe —® Cu(s) + FeSO4
A hydrometallurgical process for the extraction of metals from ores, concentrates, or secondary materials essentially contains three basic steps—dissolution of the valuable metal in the aqueous solution (leaching) purification of leach solution and subsequent recovery of metal from the purified solutions either by electrolysis or by adding some electropositive metal to it.
Some of the metals obtained by hydrometallurgy are as follows :
(A) Extraction of Ag and Au : Metals like Au and Ag can be precipitated for their salt solution by electropositive metals for example, Zn.
Metallic Ag is dissolved from its ore in dilute NaCN solution, and the solute so obtained is treated with scrap Zn when Ag is precipitated. Air is blown into the solution oxidize Na2S. Leaching the metals like silver, gold with CN– is an oxidation reaction (Ag ® Ag+ or Au ® Au+)
Ag2S (s) + 4CN– (aq) —® 2 [Ag(CN)2]– (aq) + S2– (aq)
2[Ag(CN)2]– (aq) + Zn (s) —® [Zn (CN)4]2– (aq) + 2Ag (s)
4Au (s) + 8 CN– (aq) + O2 (g) + 2H2O (l)—® 4 [Au(CN)2]– (aq) + 4OH– (aq)
2[Au(CN)2]– (aq) + Zn (s) —® [Zn(CN)4]2– (aq) + 2 Au (s)
Here Zn acts as reducing agent.
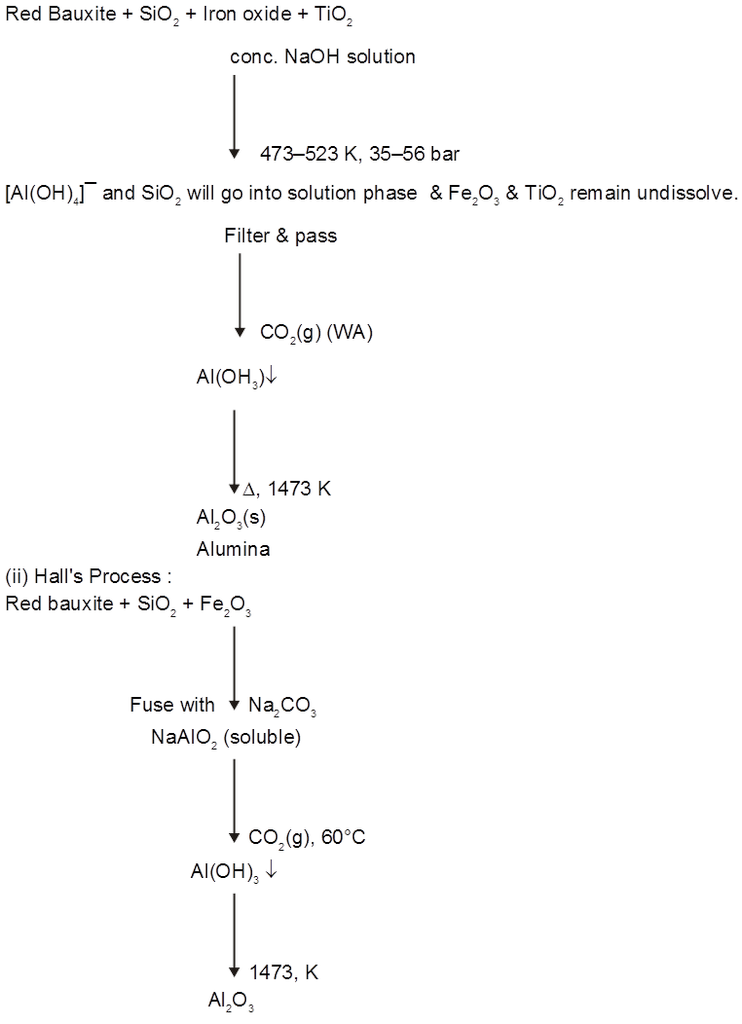
(B) Extraction of Aluminium : It involves the following processes
(a) Purification of bauxite :
(1) Leaching of Alumina from bauxite :
If Fe2O3 is main impurity (red bauxite) ® Bayer's/Hall's
If SiO2 is main impurity (white bauxite) ® Serpeck's process.
(i) Bayer's process :
(iii) Serpeck's process :
Used for white bauxite containg silica as impurities.
Al2O3 + N2 + ![]() Coke AlN + CO ↑
Coke AlN + CO ↑
![]() Al(OH)3 + NH3 ↑
Al(OH)3 + NH3 ↑
![]() Al2O3 (pure)
Al2O3 (pure)
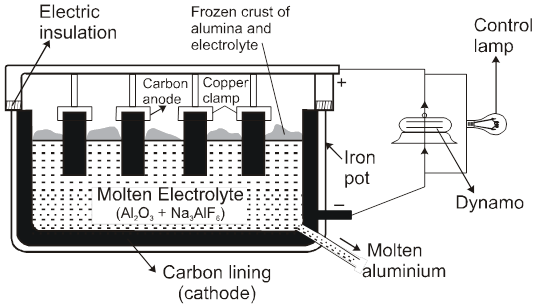
(2) Electrolytic reduction [Hall-Heroult process] :
Electrolysis of fused Al2O3 is difficult as its MP is very high (2323 K) & It is bed conductor of electricity in fused state.
So Al2O3 is mixed with Na3AlF6 or CaF2. Which lowers the MP (1173 K) & make alumina conducting.
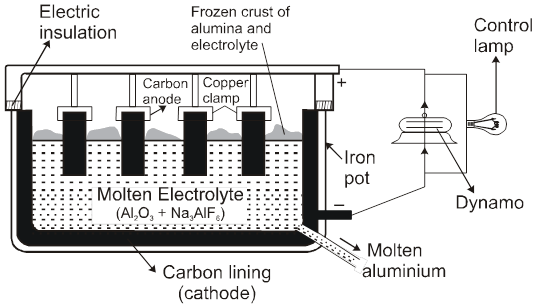
Cathode Þ Al3+ (Melt) + 3e– ® Al(l)
Anode Þ C(s) + O2– (Melt) ® CO(g) + 2e–
C(s) + 2O2– (Melt) ® CO2(g) + 4e–
Overall reaction Þ2Al2O3 + 3C ® 4Al + 3CO2
Note : During electrolytic reduction of aluminum, the carbon anodes are replaced from time to time because oxygen liberated at the carbon anodes react with anode to form CO & CO2.
Electrolytic refining of Al :
Hoop's process : For electrolytic refining of Al, Hoop's process is used.
Anode Þ Impure Al
Cathode Þ Pure Al
Electrolyte Þ Fused salt of Aluminium Fluoride
Note :The fused material remain in three different layer & remain separated because all the layer have different density.
Anode Þ Al(melt) ¾® Al3+ + 3e–
Cathode Þ Al3+ + 3e– ¾®Al
From anode (bottom layer), Al passes into solution as Al3+ ions and then from solution (middle layer) these Al3+ ion pass to cathode (top layer) and get reduced to pure metal.
Extraction of Na : The fused mixture of NaCl and CaCl2 is taken in Down’s cell which consists of circular iron cathode and carbon anode. On passing the electric current the following reactions take place :
Ionisation of NaCl : NaCl ![]() Na+ + Cl–
Na+ + Cl–
Collection of Na at cathode : Na+ + e– ® Na(Reduction).
Collection of Cl2 at anode : Cl– + e– ®Cl (Oxidation), Cl + Cl ® Cl2 .
Na can also be obtained by electrolysing molten NaOH in Castner’s cell.
Thermodynamics of extraction : Ellingham Diagram of a Metal
Ellingham Diagram
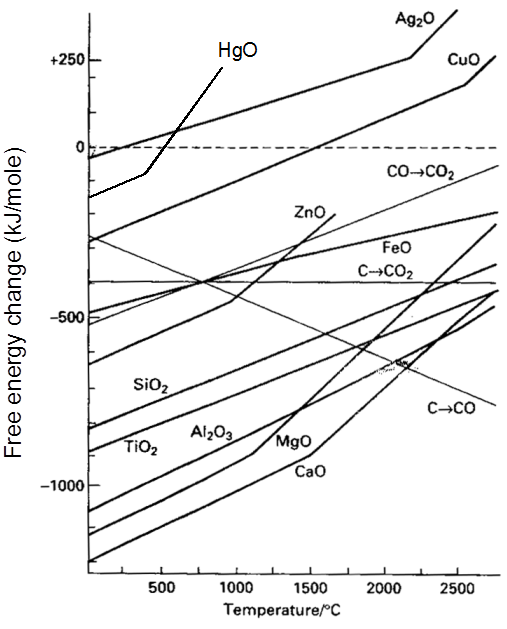
Thermodynamic of extraction :
For a Proces to be spontaneous D G must be negative.
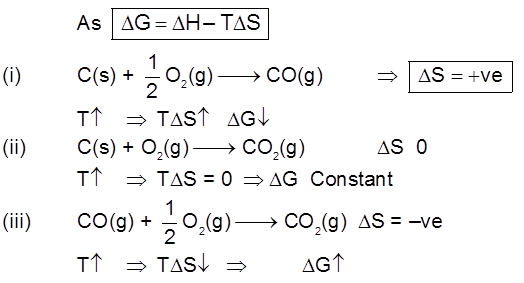
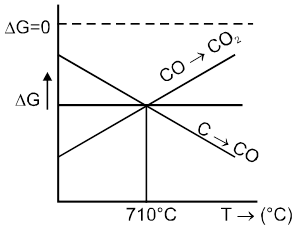
Ellingham Diagram of Metal
XM(s) + ![]() O2(g) ¾® MXOY(s)
O2(g) ¾® MXOY(s)
number of gaseous moles decreae with progress of reaction.
As DS = –ve If T Þ DG
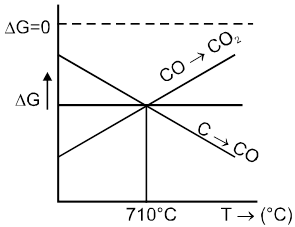
Important Points
(1) Below 710°C, CO is better reducing agent than C.
(2) All the graphs are upgrowing and on increasing temperature, DG of formation of metal oxide become less negative, while on increasing temperature, DG of C ¾® CO reaction become more negative SO, C can reduce any metal from their metal oxide.
(3) Temperature above which DG of formation of metal oxide become positive, metal oxide become unstable & it decomposes into metal & oxygen.
(4) Theoritically, all metal oxide can be decomposed to give metal & oxygen, if suficiently high temperature is applied.
(5) When plot of one metal oxide is below the others then former metal is capable of reducing the later metal oxide into their metal.
Some important result
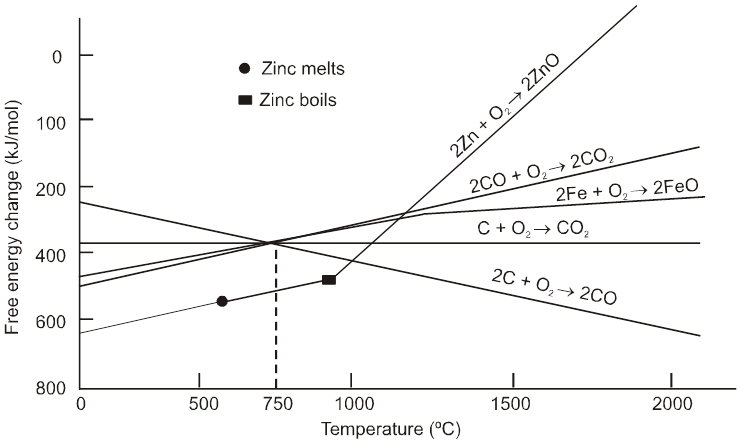
(1) All the three oxidation graph of carbon system are above the oxidation graph of ZnO upto temperature 1000°C. At this temperature C is thermodynamically capable of reducing ZnO to Zn. So C is better reducing agent for ZnO to Zn than CO.
(2) Below 1500°C temperature, the graph of MgO lies below the graph of Al2O3, so below this temperature Al cannot reduce MgO, but above this temperature graph of Al2O3 shifts below the graph of MgO so above 1500°C, Al can reduce MgO.
2. Metallurgy of Some Important Metals
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Metallurgy of Some Important Metals
1. Extraction of iron (Fe)
Ore: Haematite
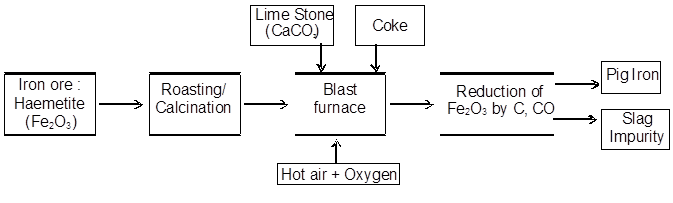
Oxide ores of iron, after concentration through calcination / roasting in reverberatory furnace (to remove water, to decompose carbonates and to oxidise sulphides) are mixed with lime stone and coke and fed into a Blast furnace from its top with the help of a cup and cone arrangement. Here, the oxide is reduced to the metal.
Thermodynamics helps us to understand how coke reduces the oxide and why this furnace is chosen. One of the main reduction steps in this process is :
FeO(s) + C(s) ® Fe(s/l) + CO (g) ........ (11)
It can be seen as a couple of two simpler reactions. In one, the reduction of FeO is taking place and in the other, C is being oxidised to CO :
FeO(s) ® Fe(s) + ![]() O2 (g) [DG(FeO, Fe) ] .............. (12)
O2 (g) [DG(FeO, Fe) ] .............. (12)
C(s) + ![]() O2 (g) ® CO (g) [DG(C, CO) ] .............. (13)
O2 (g) ® CO (g) [DG(C, CO) ] .............. (13)
When both the reactions take place to yield the equation (10), the net Gibbs energy change becomes:
D G (C,CO) + D G (FeO, Fe) = DrG .............. (14)
Naturally, the resultant reaction will take place when the right hand side in equation (14) is negative. In D G0 vs T plot representing reaction (12), the plot goes upward and that representing the change C CO (C,CO) goes downward. At temperatures above 1073K (approx.), the C,CO line comes below the Fe,FeO line
[DG(C, CO) < DG(Fe, FeO)]. So in this range, coke will be reducing the FeO and will itself be oxidised to CO. In a similar way the reduction of Fe3O4 and Fe2O3 at relatively lower temperatures by CO can be explained on the basis of lower lying points of intersection of their curves with the CO, CO2 curve in the given figure.
In the Blast furnace, reduction of iron oxides takes place in different temperature ranges. Hot air is blown from the bottom of the furnace and coke is burnt to give temperature upto about 2200K in the lower portion itself. The burning of coke therefore supplies most of the heat required in the process. The CO and heat moves to upper part of the furnace. In upper part, the temperature is lower and the iron oxides (Fe2O3 and Fe3O4) coming from the top are reduced in steps to FeO.
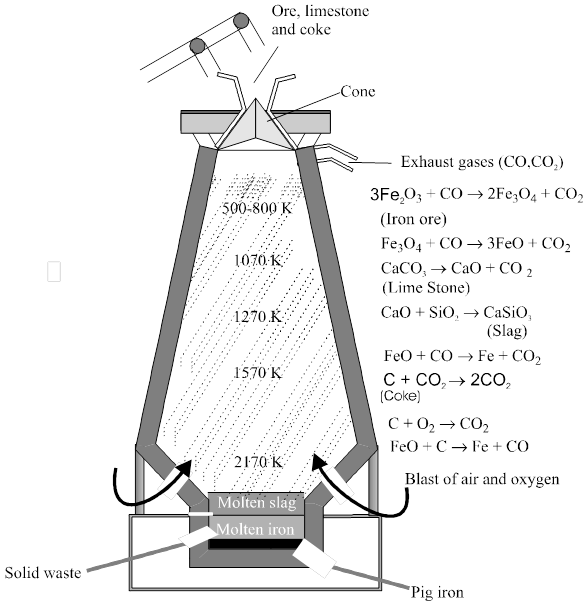
Reactions involved : The reactions proceed in several stages at different temperatures. Since the air passes through in a few seconds, the individual reactions does not reach equilibrium.
At 500 – 800 K (lower temperature range in the blast furnace)
3 Fe2O3 + CO®2 Fe3O4 + CO2
Fe3O4 + CO ® 3Fe + 4 CO2
Fe2O3 + CO ® 2FeO + CO2
At 900 – 1500 K (higher temperature range in the blast furnace):
C + CO2 ® 2 CO
FeO + CO ® Fe + CO2
Limestone is also decomposed tom CaO which removes silicate impurity of the ore as slag. The slag is in molten state and separates out from iron.
CaCO3 ® CaO + CO2
CaO + SiO2 ® CaSiO3
Note :
(1) CaSiO3 is used in cement industry or used in making building material.
(2) Slag CaSiO3 is ligther than molten Iron, get collected above it and protect iron from oxidation.
Pig Iron : Iron obtained from blast furnance contain 4%C & smaller amount of impurities of S , P , Si , Mn.
Cast Iron : Made by melting pig iron with scarp iron & coke & blast of hot air is passed through the molten mass. Contain about 3% carbon.
Wrought Iron : Purest form of iron prepared from cast iron by oxidising impurities in a reverbertory furnance lined with haematite. It have carbon about 0.1 to 0.21%
Note : (1) In steel making pure O2 is poured into molten metal where all impurities are converted to oxides then forming slag (basic oxygen) .
(2) Steel making process, from iron is an oxidation process.
(3) In production of steel from haematite ore involve first reduction & oxidation chemical process.
2. Extraction of Copper
(i) From Cuprous oxide
Sulphide ore are roasted to give oxide.
2 Cu2S + 3O2 ® 2Cu2O + 2SO2
As Cu2O line in D G V/s T graph is almost at top. so it is easily reduce to metal by heating with coke.
Cu2O + C ® 2 Cu + CO
(ii) From Copper Glance/ Copper Pyrite [self reduction]
Ore + flux  Slag + copper matte
Slag + copper matte
[CuFeS2] FeSiO3 [Cu2S + FeS]
(i) 2 CuFeS2 + 4O2 ® Cu2S + 2FeO + 3SO2.
(ii) Cu2S + FeO + SiO2 ® FeSiO3 + Cu2S.
Fusible slag Matte
* Chemical composition of matte = (Mostly Cu2|S + some FeS)
2 FeS + 3O2 ® 2 Fe + 2 SO2 ,
FeO + SiO2 ® FeSiO3 [Slag]
Cu2S + 3O2 ® 2 Cu2O + 2 SO2
2 Cu2O + Cu2S ® 6 Cu + SO2 [Self Reduction ]
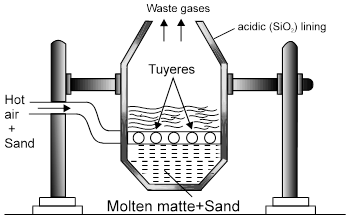
Note : Solidified copper has blistered appaerance due to evolution of SO2 so called as blister copper.
(iii) From Low Grade Ore
Þ From low grade ore , Cu is extracted by hydrometallurgy. (leaching)
(a) Low grade ore of Cu
Cu2O (Cuprite)
Cu2S [Copper glance] + ![]() CuSO4
CuSO4
Cu(OH)2 . CuCO3 (Malachite)
(b) CuSO4 ![]() Cu
Cu
(i) Electrolysing or
(ii) Metal displacement with Fe
Electrolytic Refining [Cu is purified by electro refining]
Anode Þ impure Cu & cathode = pure Cu.
Electrolyte Þ aqueous solution of CuSO4 :
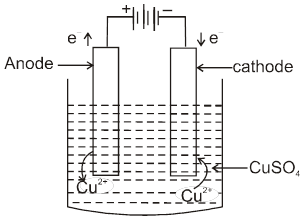
Anode Þ Cu(s) ® Cu2+(aq) + 2e–
Cathode Þ Cu2+(aq) + 2e ®Cu(s)
Anode Mud Þ Ag , Au , Pt.
3. Extraction of lead :
Ore: PbS (Lead sulphide)
There are two methods of extracting the element :
(i) Roast in air to give PbO, and then reduce with coke or CO in a blast furnace.
2PbS(s) + 3O2 (g) ![]() 2PbO (s)
2PbO (s) 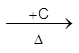 2Pb(l) + CO2 (g)
2Pb(l) + CO2 (g)
(ii) PbS is partially oxidized by heating and blowing air through it. After some time the air is turned off and heating is continued. The mixture undergoes self reduction as given below.
3PbS(s) 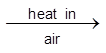 PbS (s) + 2PbO (s)
PbS (s) + 2PbO (s) ![]() 3Pb(l) + SO2 (g)
3Pb(l) + SO2 (g)
Chart3:
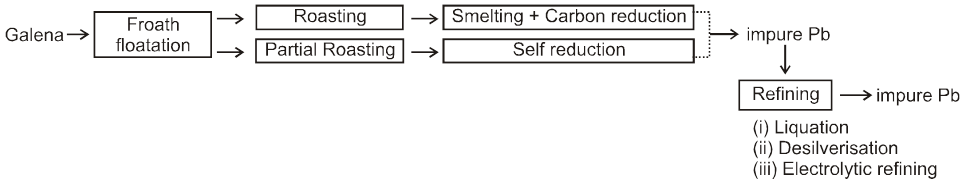
4. Extraction of zinc :
Ore: ZnS (Zinc blende)
The ore is roasted in presence of excess of air at temperature 1200 K.
2 ZnS + 3O2 ® 2 ZnO + 2SO2
The reduction of zinc oxide is done using coke. The temperature in this case is higher than that in case of copper. For the purpose of heating, the oxide is made into brickettes with coke and clay.
ZnO + C ![]() Zn + CO
Zn + CO
The metal is distilled off and collected by rapid chilling.
Note :
ZnO may be reduced by carbon monoxide at 1473 K (i.e. 1200°C) in smelter. The reaction is reversible, and the high temperature is required to move the equilibrium to the right. At this temperature the Zn is gaseous. If the gaseous mixture of Zn and CO2 was simply removed fom the furnace and cooled, then reoxidation of Zn would occur. Thus the zinc powder obtained would contain large amounts of ZnO.
ZnO + CO ![]() Zn + CO2
Zn + CO2
Chart4 :
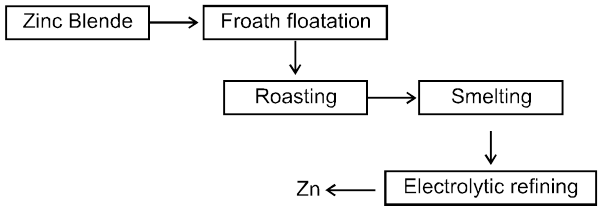
5. Extraction of tin from cassiterite (SnO2)
(i) Washing with water Þ To remove silicious impurities.
(ii) Electromagnetic sepration Þ To remove magnetic impurity of wolframite.
(iii) Roasting
(a) To remove volatile impurities S as SO2 As as AS2O3 and Sb as Sb2O3.
(b) ore contain impurites of CuS & FeS which are converted to their sulphate
CuS + 2O2 ¾® CuSO4, FeS + 2O2 ¾®FeSO4
(iv) Leaching ® CuSO & FeSO4 dissolve in water.
(v) Washing ® Ore is washed with running water. obtained orecontain 60-70% SnO2 & called as Black Tin.
(vi) Smelting : SnO2 is reduced by Carbon.
Product contain traces of Fe which is removed by passing air through molten mixture.
SnO2 + C ¾® Sn + CO
2Fe + O2 ¾®2FeO
Chart5:
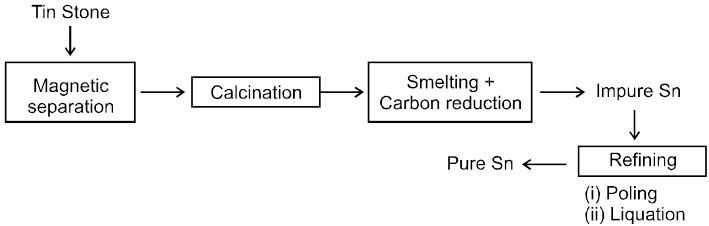
6. Extraction of Magnesium :
(i) From Carnallite :
The ore is dehydrated in a current of hydrogen chloride and the mixture of fused chlorides is electrolysed.
(ii) From Sea water (Dow’s process) :
Sea water contains 0.13% magnesium as chloride and sulphate. It involves following steps.
(a) Precipitation of magnesium as magnesium hydroxide by slaked lime :
MgCl2 + Ca(OH)2 ® Mg(OH)2![]() + CaCl2
+ CaCl2
(b) Preparation of hexahydrated magnesium chloride :
Mg(OH)2 + 2HCl(aq) ® MgCl2 + 2H2O
The solution on concentration and crystallisation gives the crystals of MgCl2.6H2O
(c) Preparation of anhydrous magnesium chloride :
MgCl2. 6H2O ![]() MgCl2 + 6H2O
MgCl2 + 6H2O
It is not made anhydrous by simple heating because it gets hydrolysed
MgCl2. 6H2O ![]() MgO + 5H2O + 2HCl
MgO + 5H2O + 2HCl
(d) Electrolysis of fused anhydrous MgCl2 :
Magnesium chloride obtained by any of the above methods is fused and mixed with sodium chloride and calcium chloride in the temperature range of 973 – 1023 K. The molten mixture is electrolysed. Magnesium is liberated at the cathode (iron pot) and chlorine is evolved at graphite anode.
MgCl2 ![]() Mg2+ + 2Cl–
Mg2+ + 2Cl–

At cathode : Mg2+ + 2e– ® Mg(99% pure) ;
At anode : 2Cl–® Cl2 + 2e–
A stream of coal gas is passed through the pot to prevent oxidation of magnesium metal. The magnesium obtained in liquid state is purified by distillation under reduced pressure.
(1 mm of Hg at 873 K).
7. Extraction of gold and silver (MacArthur-Forrest cyanide process) :
Chart6 :
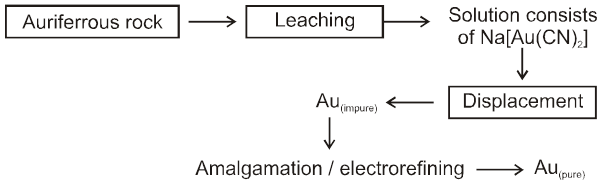
Chart7:
8. Extraction of Gold & Silver
MacArthur-forrest Cyanide process
(A) From native ore :
(i) Complex formation with CN¯ in presence of air
Ag + CN¯ + O2 ¾® [Ag(CN)2]¯(aq)
In this process, air is used which oxidises metal M(Ag/Au) to M+(Ag+/Au+) which form complex with CN¯ ion.
(ii) Metal displacement with Zn
[Ag(CN)2]¯ + Zn ¾® [Zn(CN)4]2–(aq) + Ag(s)
This is leaching (Hydro Meltallurgy) process.
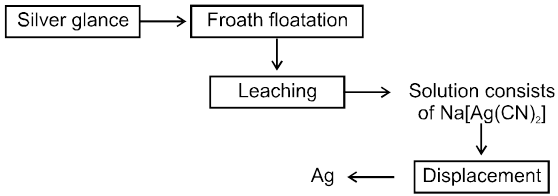
(B) From Argentite ore
Extraction of Ag from Argentite ore by dissolving in NaCN and then using metal displacement is leaching process,which is an example of hydrometallurgy.
Ag2S (conc. ore) + 2 NaCN ![]() 2AgCN + Na2S
2AgCN + Na2S
Role of air : As Ag2S & AgCN are in equilibrium , air oxidise
Na2S to Na2SO4 & shift the equilibrium right.
4 Na2S + 5O2 + 2H2O ® 2Na2SO4 + 4 NaOH + 2S
AgCN + NaCN ® Na [Ag(CN)2] " soluble complex "
2 Na [Ag(CN)2] + Zn [dust] ® 2 Ag + Na [Zn (CN)4]
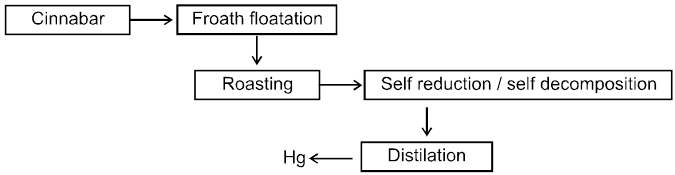
9. Extraction of Mercury
Chart8:
3. Purification or Refining of Metal
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Purification or Refining of Metal
Physical Method :
(1) Liquiation Process :
Based on difference in melting point of metal and impurity. This process is used for purification of those metal which have low melting point than each of impurities associated with metal.
Used for Purification of
(i) Impure tin metal (ii) Impure zinc (spelter)
Spelter Þ 97 % to 98 % zinc is known as spelter.
(iii) For removing Pb from Zn – Ag alloy.
(2) Fractional Distillation process
Used for purifying those metal which are easily volatile, while impurities present in it are not used for purifying Zn , Cd & Hg.
(3) Zone refining of metal
Principle : Based on principle that an impure molten metal on gradually celling will deposit crystal of pure metal while the impurity will be left in the remaining part of molten metal.
*This method is used when metal is required in very high purity.
Mainly used for purification of Si & Ge.
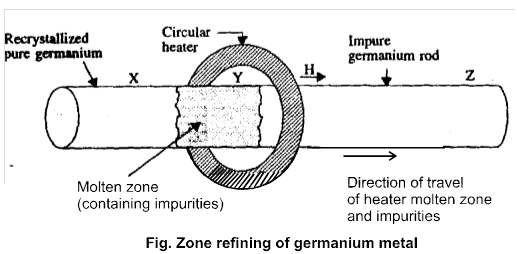
Note : High purity of metal can be obtained by zone refing method , if impurities have lower melting point.
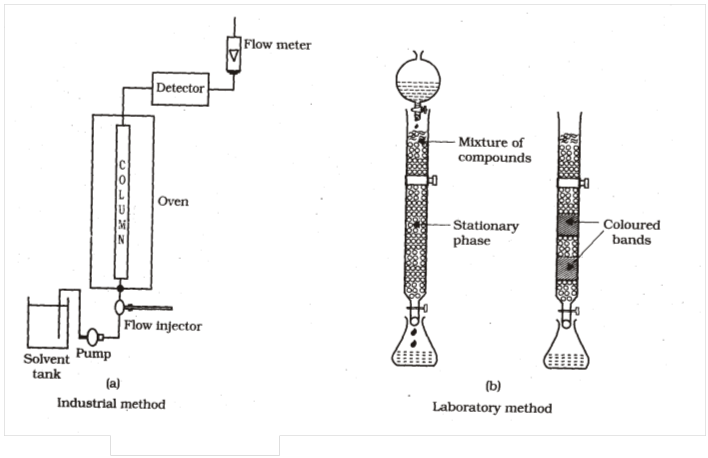
(4) Chromatographic method
Principle : Based on the principle of adsorption.
*Different component of a mixture are differently adsorbed on an adsorbent.
Chemical Method
(1) Oxidative Refining
Used when impurities present in metal are easily oxidised by oxygen.
Mainly used for refining Þ Pb , Ag , Cu , Fe .....
In this method , molten impure metal is subjected to oxidation.
(i) Bessemerisation [Purification of Iron]
Mainly used for manufacture of steel from cast iron.
Haematite ore  pig iron
pig iron 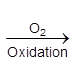 steel
steel
P is also oxidise to P4O10
P4 (l) + 5O2 ® P4O10 (l)
6 CaO (g) + P4O10 (l) ® 2 Ca3 (PO4)2 (l)
Thomas Slag
Which is used as fertilizer.
(ii) Cupellation (Removal of lead)
Mainly used to remove Pb from Ag or Au.
(2) Poling Process
This method is used for purification of those impure metal which contain their own oxide as one of the impurities.
Eg. (a) Purification of Cu.(b) Purification of impure Tin.
(3) Electrolytic refining
Metal , Cu , Ni & Al refined electrolytically.
The anode mud obtained in the electrolytic refining has
Electrolytic refining Anode Mud
(i) Lead Ag , Au , Sb , Cu.
(ii) Cu Ag , Au , Pt.
(iii) Ag Au , Pt.
(4) (a) Kroll's Process Þ For Ti & Zr.
TiCl4 + 2 Mg ![]() Ti + 2MgCl2
Ti + 2MgCl2
(b) IMI process Þ [ Imperial Metal industries ]
TiCl4 + 4 Na 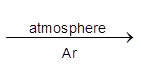 Ti + 4 NaCl.
Ti + 4 NaCl.
Vapour phase refining :
(a) Extraction of Nickel [ Mond's Process ]
Used for purification (refining) of Ni.
Ni form complex with Co , [Ni(CO)4] which volatilized
[BP=43°] & then decompose at 200°C in Ni & CO.
Ni(S) + 4 CO ![]() Ni (CO)4(g)
Ni (CO)4(g) ![]() Ni + 4 CO.
Ni + 4 CO.
readily volatile
(B.P. = 43°C)
(b) VanArkel – DeBoer Process
Used for purification of Ti , Zr , Bi & B.
Small amount of puremetal is obtained.
Impure Ti(S) + 2I2 ![]() TiI4(g)
TiI4(g) ![]() Ti + 2I2
Ti + 2I2
KROLL’S PROCESS :
TiCI4 + 2 Mg ![]() Ti + 2 MgCI2 (Kroll’s process)
Ti + 2 MgCI2 (Kroll’s process)
TiCI4 + 4 Na 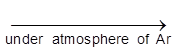 Ti + 4 NaCI (Imperial metal industries (IMI) process)
Ti + 4 NaCI (Imperial metal industries (IMI) process)
NaCI is leached with H2O. Ti is in the form of small granules. These can be fabricated into metal parts using “powder forming” techniques and sintering in an inert atmosphere. Zr is also produced by Kroll’s process.
VAPOR PHASE REFINING :
(i) Extraction of Nickel (Mond’s process) :
Nickel is extracted from sulfide ore by roasting followed by reduction with carbon, but the process is complicated by the fact that nickel is found in association with other metals. The refining is rather unusual, for nickel forms a complex with carbon monoxide tetracarbonylnickel (O) [Ni(CO)4]. This substance is molecular in molecular in structure and readily volatilized (boiling point 43ºC). It is made by heating nickel powder to 50ºC, in a stream of CO and then decomposed at 200ºC. Any impurity in the nickel sample remains in the solid state and the gas is heated to 230ºC, when it decomposes, giving pure metal and CO, which is recycled. Ni(CO)4 is gaseous and may be produced by warming nickel with CO at 50ºC.
The sequence of reaction is
H2O(g) + C ® CO(g) + H2
Ni(s) + 4 CO(s) ![]() [Ni(CO4)] (g)
[Ni(CO4)] (g)
[Ni (CO)4](g) ![]() Ni + 4CO(g)
Ni + 4CO(g)
(ii) Van Arkel–De Boer process :
Small amounts of very pure metals (Ti, Zr, or Bi) can be produced by this method. This process is based on the fact that iodides are the least stable of the halides. The impure element is heated with iodine, producing a volatile iodide, TiI4, ZrI4, or BiI3. These are decomposed by passing the gas over an electrically heated filament of tungsten or tantalum that is white hot. The element is deposited on the filament and the iodine is recycled. As more metal is deposited on the filament, it conducts electricity better. Thus, more electric current must be passed to keep it white hot. Thus the filament grows fatter and eventually the metal is recovered. The tungsten core is distilled out of the center and a small amount of high purity metal is obtained.
Impure ![]()
The method is very expensive and is employed for the preparation of very pure metal for specific use.
(iii) Parke’s process :
The removal of the impurities of Ag from the commercial lead is called desilverisation of lead and is done by Parke’s process . Thus, Parke’s process is the desilverisation of lead.
In Parke’s process, the commercial lead, which contains Ag as impurities, is melted in iron pots and 1% of Zn is added to it. The molten mass is thoroughly agitated. Since Ag is about 300 times more soluble in Zn than in Pb, most of the Ag present in the commercial lead as impurity mixes with Zn, to form Zn–Ag alloy. When the whole is cooled, two layers are obtained. The upper layer contains Zn–Ag alloy in the solid state, while the lower layer has lead in the molten state. This lead contains only 0.0004% of Ag and hence is almost pure. Lead obtained after removing most of Ag from it (desilverisation of lead) by Parke’s process, is called desilverised lead. This lead contains the impurities of metals like Zn, Au, Sb etc. These metal impurities are removed from desilverised lead by Bett’s electrolytic process.
Zn–Ag alloy, formed in the upper layer, is skimmed off from the surface of the molten lead by perforate ladles. This alloy contains lead as impurity. This impurity of Pb is removed from the alloy by liquation process, in which Zn–Ag alloy is heated in a slopping furnace, when the impurity of Pb melts and hence drains away from the solid alloy. Thus purified Zn–Ag is obtained. Now Ag can be obtained from this purified Zn–Ag alloy by distillation process, in which the alloy is heated strongly in presence of little carbon in a fire–clay retort. Zn, being more volatile, distills off while Ag remains in the retort, carbon used in the process reuses the oxide of Zn, if formed. Ag obtained from Zn–Ag alloy is contaminated with a little of Pb as impurity. This impurity of Pb placed in a cupel (cupel is a boat–shaped) dish made of bone ash which is porous in nature) in a reverberatory furnace and heated in the presence of air. By doing so, lead (impurity) is oxidised to PbO(litharge) which volatilises and pure Ag is left behind in the cupel. Last traces of PbO are absorbed by the porous mass of the cupel.
(iv) Pudding process : This process is used for the manufacture of wrought iron from cast iron. We know that cast iron contains the impurities of C, S, Si, Mn and P. When these impurities are removed from cast iron, we get wrought iron. In this process the impurities are oxidised to theiro xides not by blast of air but by the haematite (Fe2O3) lining of the furnace.
1. Occurrence of metals
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chapter 6
General principles and processes of isolation of elements
Introduction :
The compound of a metal found in nature is called a mineral. The minerals from which metal can be economically and conveniently extracted are called ores. An ore is usually contaminated with earthy or undesired materials known as gangue. So all minerals are not ores but all ores are minerals. Ores may be classified mainly into following four classes.
(a) Native ores : They contain the metal in free state. Silver, gold, platinum etc, occur as native ores.
(b) Oxidised ores : These ores consist of oxides or oxysalts (e.g. carbonates, phosphates, sulphates and silicates ) of metals.
(c) Sulphurised ores : These ores consist of sulphides of metals like iron, lead, zinc, mercury etc.
(d) Halide ores : These ores consist of halides of metals.
Important ore :
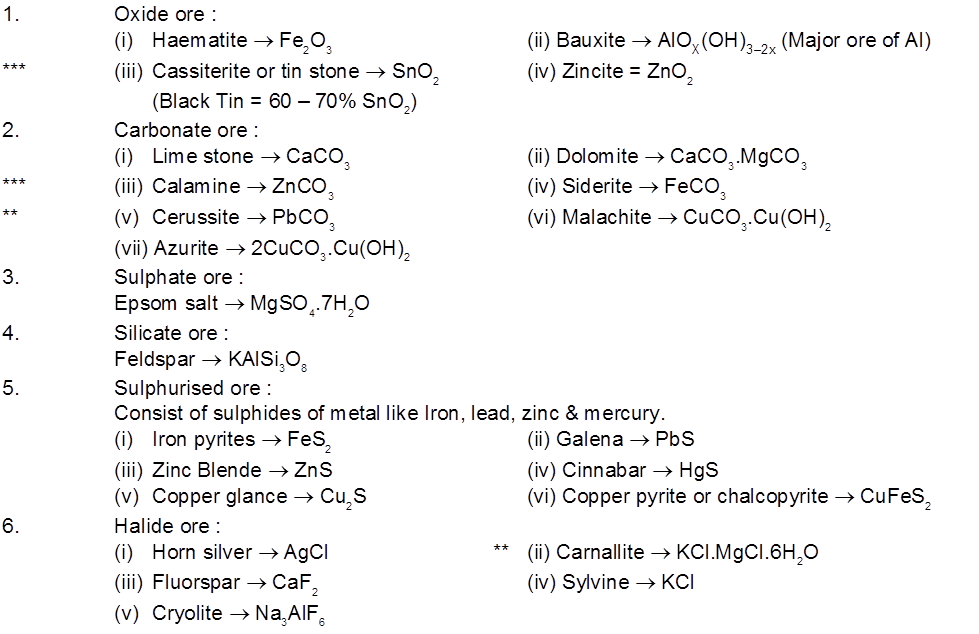
Note : Mg obtained from both sea water & earth crust.
Metallurgy :
The scientific and technological process used for the extraction/isolation of the metal from its ore is called as metallurgy.
The isolation and extraction of metals from their ores involve the following major steps:
(A) Crushing of the ore.
(B) Dressing or concentration of the ore.
(C) Isolation of the crude metal from its ore
(D) Purification or refining of the metal.
Chart1:
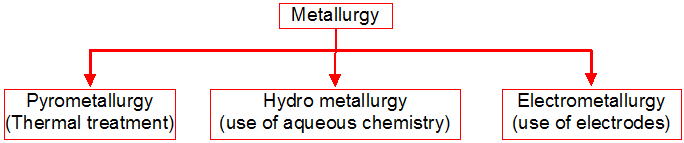
Chart2: Steps involved in metallurgy.
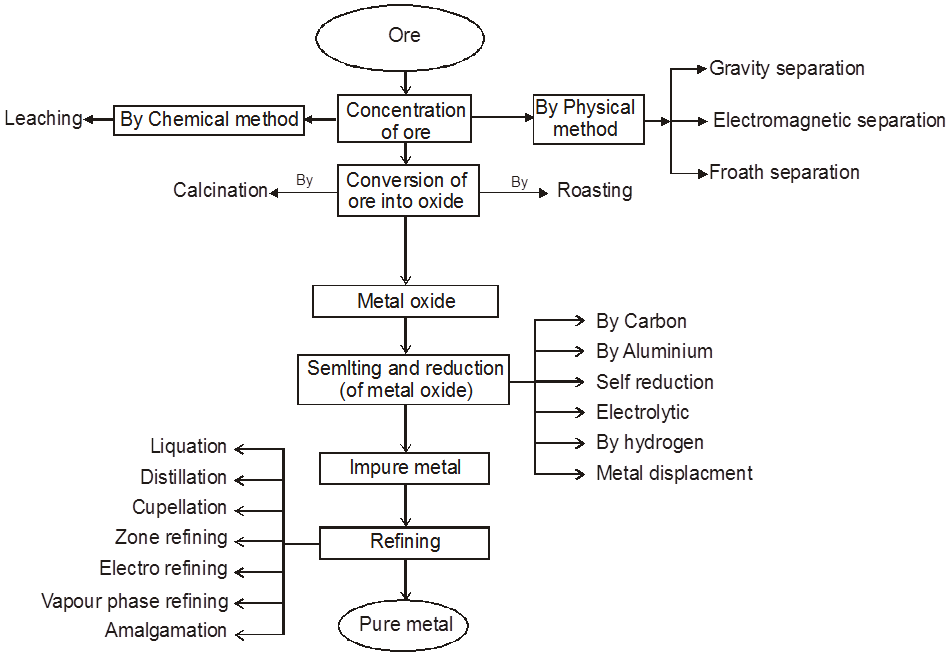
1. Physical Method :
(A) Crushing and Grinding : The ore is first crushed by jaw crushers and ground to a powder (pulverisation of the ore) in equipments like ball mills and stamp mills.
(B) Concentration : The removal of unwanted useless impurities from the ore is called dressing, concentration or benefaction of ore.
It involves several steps and selection of these steps depends upon the difference in physical properties of the compound of metal and that of gangue. Some of the important procedures are described below.
(i) Hydrolytic washing :
Gravity separation or "Levigation". Based on the difference in the densities of the gangue and ore particle.
Generally used for the concentration of oxide & native ore.
(ii) Electromagnetic sepration :
Based on difference in magnetic properties of mineral and gangue particle.
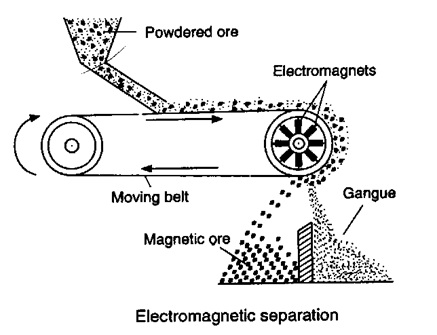
(a) Chromite ore [FeO.Cr2O3] is seprated from non magnetic silicious impurities.
(b) Cassiterite ore [SnO2] is seprated from magnetic wolframite [FeWO4 + MnWO4]
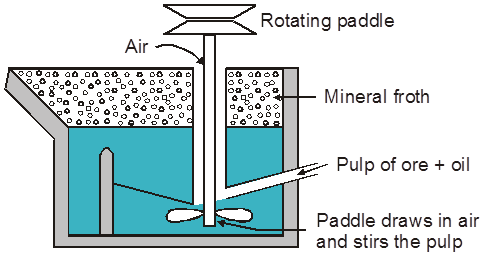
(iii) Froth floatation process :
Generally used for concentration of low grade sulphide ore PbS, ZnS, Cu2S, CuFeS2
Principle :
Based on fact that mineral & gangue particles have different wettability in water and oil (pine oil used)
Mineral particle ® are wetted by oil.
Gangue particle ® Wetted by water.
Reagents Used :
(i) Frothers :
These form stable froth which rises to the top of the flotation cell.
Oil like pine oil, comphor oil are used in small quantities.
Main Function of :
Frother ®Stick to ore & then take it to rise upto the top.
Stabilizer ® To stabilize the froth, froth stabilizer like [cresol & aniline] are added.
(ii) Collector :
K+ or Na+ ethyl xanthates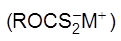 (R = alkyl,M+ = Na+ , K+) are used as collectors. Which collect or attract mineral partical and make them water repellant.
(R = alkyl,M+ = Na+ , K+) are used as collectors. Which collect or attract mineral partical and make them water repellant.
Main Function : Make the ore hydrophobic.
(iii) Activating & Depressing agents :
In PbS + ZnS + FeS2 mixture NaCN + Na2CO3 is used to depress the floation properties of ZnS & FeS2.
ZnS + CN– ¾® [Zn(CN)4]2–
FeS2 + CN– ¾® [Fe(CN)6]4–
CuSO4 is then added which is activating for ZnS as Cu forms more stable complexing with CN– than Zn2+
2. Chemical Method :
(4) Leaching :
Used when ore is soluble in some suitable solvent like ® acid, base & suitable chemical reagent.
Ex. (a) Leaching of alumina from bauxite.
(b) Extraction of Ag & Au from their ores in the complex form by treatment with NaCN & KCN.
(c) Treatment of low grade Cu ores with conc. H2SO4.
(C) Extraction of crude metal from concentrated ore :
The concentrated ore must be converted into a form which is suitable for reduction. Usually the sulphide ore is converted to oxide before reduction. Oxides are easier to reduce. Thus isolation of metals from concentrated ore involves two major steps as given below.
(i) Conversion to oxide
(ii) Reduction of the oxide to metal.
(i) Conversion to oxide :

Conversion of ore into oxide is carried out in two ways depending upon the nature of ore.
Calcination. It is a process of heating the concentrated ore strongly in a limited supply of air or in the absence of air. The process of calcination brings about the following changes :
(a) The carbonate ore gets decomposed to form the oxide of the metal, e.g.,
FeCO3 (siderite)  FeO + CO2
FeO + CO2
PbCO3 (cerrussite)  PbO + CO2
PbO + CO2
CaCO3 (calcite ore / lime stone)  CaO + CO2
CaO + CO2
ZnCO3 (calamine)  ZnO + CO2
ZnO + CO2
CuCO3.Cu(OH)2 (malachite)  2CuO + H2O + CO2
2CuO + H2O + CO2
MgCO3.CaCO3 (dolomite)  MgO + CaO + 2CO2
MgO + CaO + 2CO2
(b) Water of crystallisation present in the hydrated oxide ore gets lost as moisture, e.g.,
2Fe2O3.3H2O (limonite)  2Fe2O3(s) + 3H2O(g)
2Fe2O3(s) + 3H2O(g)
Al2O3. 2H2O (bauxite)  Al2O3 (s) + 2H2O(g)
Al2O3 (s) + 2H2O(g)
c) Organic matter, if present in the ore, gets expelled and the ore becomes porous. Volatile impurities are removed.
Roasting :
Generally used for sulphide ore.
Process : Concentrated ore is strongly heated in excess of air or O2 below its metling point.
(a) Roasting at moderate temperature :
2PbS + 3O2  2PbO + 2SO2
2PbO + 2SO2
2ZnS + 2O2  2ZnO + 2SO2
2ZnO + 2SO2
If temperature is low (500°C) & concentration of SO2 is high sulphate are produced.
PbS + 2O2  PbSO4
PbSO4
ZnS + 2O2  ZnSO4
ZnSO4
(b) Roasting at High temperature :
Self reduction / auto reduction / air reduction :
Sulphide ore of Cu, Pb, Hg & Sb when strongly heated in free supply of air, directly reduced to the metal this known as self reduction.
Cu2S (Copper glance) + O2 ¾® 2Cu + SO2
PbS (Gelena) + O2 ¾® Pb + SO2
HgS (Cinabar) + O2 ¾® Hg + SO2
Important Points
1.It remove impurities of As as As2O3, sulphur as SO2, P as P4O10 & Sb as Sb2O3
4M (M = As, Sb) + 3O2 ¾®2M2O3↑
S + O2 ¾®SO2↑
P4 + 4O2 ¾® P4O10↑
2. Impurities of CuS & FeS in SnO2 converted to CuSO4 & FeSO4
CuS + 2O2 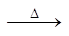 CuSO4
CuSO4
FeS + 2O2 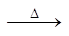 FeSO4
FeSO4
Note : Calcination & Roasting carried out in a reverberatory furnace.
Smelting :
Slag formation : In many extraction processes, an oxide is added deliberately to combine with other impurities and form a stable molten phase immiscible with molten metal called a slag. The process is termed smelting.
The principle of slag formation is essentially the following :
Nonmetal oxide (acidic oxide) + Metal oxide (basic oxide) ¾® Fusible (easily melted) slag
Removal of unwanted basic and acidic oxides: For example, FeO is the impurity in extraction of Cu from copper pyrite.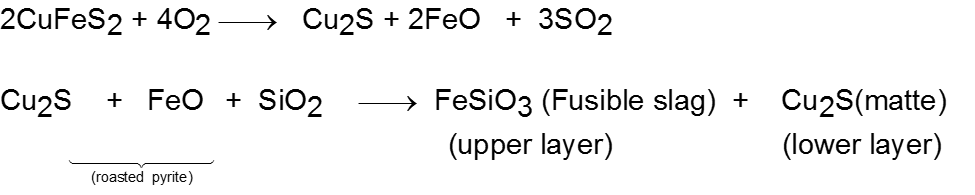
Matte also contains a very small amount of iron(II) sulphide.
To remove unwanted acidic impurities like sand and P4O10, smelting is done in the presence of limestone.
CaCO3 ¾® CaO + CO2
CaO + SiO2 ¾® CaSiO3 (fusible slag)
6CaO + P4O10 ¾® 2Ca3(PO4)2 (fusible slag - Thomas slag)
Properties of a slag :
(i) Slag is a fusible mass.
(ii) It has low melting point.
(iii) It is lighter than and immiscible with the molten metal. It is due to these impurities that the slag floats as a separate layer on the molten metal and can thus be easily separated from the metal. The layer of the slag on the molten metal prevents the metal from being oxidised.
Type of flux : Fluxes are of two types viz., acidic flux and basic flux.
(a) Acidic flux : It is an acidic oxide (oxide of a non-metal) like SiO2, P2O5, B2O3 (from borax). It is used to remove the basic impurity like CaO, FeO, MgO etc. The acidic flux combines with the basic impurity and forms a slag.
(b) Basic flux : It is a basic oxide (i.e., oxide of a metal) like CaO (obtained from lime stone, CaCO3), MgO (from magnesite, MgCO3), haematite (Fe2O3) etc. It is used to remove the acidic impurity like SiO2, P2O5 etc. The basic flux combines with the acidic impurity and forms a slag.
Thus, slag can be defined as a fusible mass, which is obtained when a flux reacts with an infusible acidic or basic impurity present in the oxide ore.
Reduction of a Metal Oxide
(1) Reduction with Carbon :
PbO + C ¾® Pb + CO
2Fe2O3 + 3C ¾® 4Fe + 3CO2
*** ZnO + C 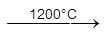 Zn + CO (Extraction of Zn)
Zn + CO (Extraction of Zn)
*** SnO2 + 2C 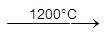 Sn + 2CO
Sn + 2CO
(2) Reduction with CO :
Fe2O3 + 3CO ¾® 2Fe + 3CO2
Fe3O4 + 4CO ¾® 3Fe + 4CO2
Reduction with C & CO is carried out in blast furnace.
(3) Redution with Al :
GoldSchmidt or Aluminothermic process :
Metallic oxide of Cr & Mn reduced by Al & this reaction is known as thermite reaction.
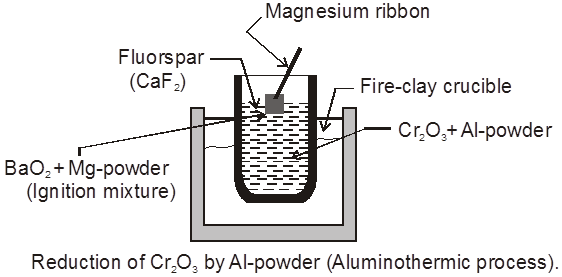
Mg + BaO2 ¾® BaO + MgO + Heat
(i) Cr2O3 + Al ¾®2Cr(l) + Al2O3
*** (ii) 3Mn3O4 + 8Al ¾® 4Al2O3 + 9Mn
(iii) 2Al + Fe2O3 ¾®Al2O3 + 2Fe
(iv) B2O3 + 2Al ¾® 2B + Al2O3
(4) Redution by Mg & Na :
(i) TiCl4 + 2Mg 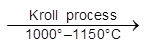 Ti + 2MgCl2
Ti + 2MgCl2
(ii) TiCl4 + 4Na 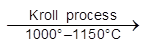 Ti + 4NaCl
Ti + 4NaCl
(5) Self reduction or auto reduction or air reduction :
Sulphide ore of some of the metal like Hg, Cu, Pb & Sb, when heated in air some part of these ore change into oxide or sulphate, thenthat part react with remaing part of sulphide ore to give its metal & sulphur dioxide.
This process is known as self reduction.
Ex. (i)(a) 2PbS + 3O2 ¾®2PbO + 2SO2
2PbO + PbS ¾®3Pb + SO2
(b) PbS + 2O2 ¾®PbSO4
PbSO4 + PbS ¾®2Pb + 2SO2
(ii) 2HgS + 3O2 ¾®2HgO + 2SO2
2HgO + HgS ¾®3Hg + SO2
(6) Electrolytic Reduction :
Electrolytic reduction is an expensive method than chemical method, that's why generally we do not use this method.
But when very high pure metal is required then we use this method.
This method is also used for highly reactive metal.
(i) Electrolyric reduction in aqueous solution :
This method is used when product does not react with water.
Electrolytic reduction of Cu & Zn from their sulphates.
(ii) In other solvents : Flourine react with water so it is produced by electrolysis of KHF2 dissolved in anhydrous HF.
(iii) In fused metal : When produced metal react with water, then metal is extracted from fused melt of their ionic salt.
Eg. (i) Al is extracted from electrolysis of fused mixture of Al2O3 and cryolite (Na3AlF6).
(ii) Extraction of Na by electrolysis of fused NaCl.
Note : In this electrolysis CaCl2 is added as impurity to lower the melting point from 800°C to 500°C.
(i) Hydro Metallurgy :
When metal can be extracted using solution without any heating or without any electrolysis then operation is known as hydrometallurgy.
Basic Step
(i) Dissolution of the valuable metal in aqueous solution.
(ii) Purification of leach solution.
(iii) Recovery of metal from purified solution.
Ex. Extraction of Ag & Au :
Metallic Ag dissolved in NaCN from ore of Ag & then precipitated with the help of Zn.
AgS(s) + 4CN¯ ¾® 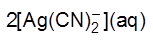 + S2–
+ S2–
2[Ag(CN)2]¯(aq) + Zn(s) ¾® [Zn(CN)4]2–(aq) + 2Ag(s)
(ii) Pyrometallurgy :
If furnace are used and ore are heated to extract metal then it is called pyrometallurgy.
Electrochemical principles of metallurgy :
Electrolytic reduction can be regarded as a technique for driving a reduction by coupling it through electrodes and external circuit to a reactive or a physical process with a more negative DG. The free energy available from the external source can be assessed from the potential it produces across the electrodes using the thermodynamic relation :
DG = –nFE ..........(i)
where n is the number of electrons transferred, F is Faraday’s constant (F = 96.5 kJ/mol) and Eº is electrode potential of the redox coupled formed in the system.
Hence, the total Gibb’s energy of the coupled internal and external process is
DG + DG (external) = DG – nFEext
If the potential difference of the external source exceeds
Eext = –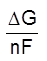
the reduction is thermodynamically feasible; thus, the overall process occurs with a decrease in free energy.
More reactive metals have large negative values of the electrode potential. So their reduction is difficult. If the difference of two E0 values corresponds to a positive E0 and consequently negative DG0 in equation (i), then the less reactive metal will come out of the solution and the more reactive metal will go to the solution, e.g.,
Cu2+ (aq) + Fe(s) —® Cu(s) + Fe2+(aq)
In simple electrolysis, the Mn+ ions are discharged at negative electrodes (cathodes )and deposited there. Precautions are taken considering the reactivity of the metal produced and suitable materials are used as electrodes. Sometimes a flux is added for making the molten mass more conducting.
Hydrometallurgy : The processing of ores and minerals as well as metals and their compounds at relatively low, often ambient temperatures employing aqueous solution is known as hydrometallurgy. Occasionally, organic reagents are also used. This method of extraction is generally used for low grade ores. Copper is extracted by hydrometallurgy from low grade ore it is leached out using acid and bacteria. The solution containing Cu2+ is treated with scrap iron or H2.
CuSO4 + Fe —® Cu(s) + FeSO4
A hydrometallurgical process for the extraction of metals from ores, concentrates, or secondary materials essentially contains three basic steps—dissolution of the valuable metal in the aqueous solution (leaching) purification of leach solution and subsequent recovery of metal from the purified solutions either by electrolysis or by adding some electropositive metal to it.
Some of the metals obtained by hydrometallurgy are as follows :
(A) Extraction of Ag and Au : Metals like Au and Ag can be precipitated for their salt solution by electropositive metals for example, Zn.
Metallic Ag is dissolved from its ore in dilute NaCN solution, and the solute so obtained is treated with scrap Zn when Ag is precipitated. Air is blown into the solution oxidize Na2S. Leaching the metals like silver, gold with CN– is an oxidation reaction (Ag ® Ag+ or Au ® Au+)
Ag2S (s) + 4CN– (aq) —® 2 [Ag(CN)2]– (aq) + S2– (aq)
2[Ag(CN)2]– (aq) + Zn (s) —® [Zn (CN)4]2– (aq) + 2Ag (s)
4Au (s) + 8 CN– (aq) + O2 (g) + 2H2O (l)—® 4 [Au(CN)2]– (aq) + 4OH– (aq)
2[Au(CN)2]– (aq) + Zn (s) —® [Zn(CN)4]2– (aq) + 2 Au (s)
Here Zn acts as reducing agent.
(B) Extraction of Aluminium : It involves the following processes
(a) Purification of bauxite :
(1) Leaching of Alumina from bauxite :
If Fe2O3 is main impurity (red bauxite) ® Bayer's/Hall's
If SiO2 is main impurity (white bauxite) ® Serpeck's process.
(i) Bayer's process :
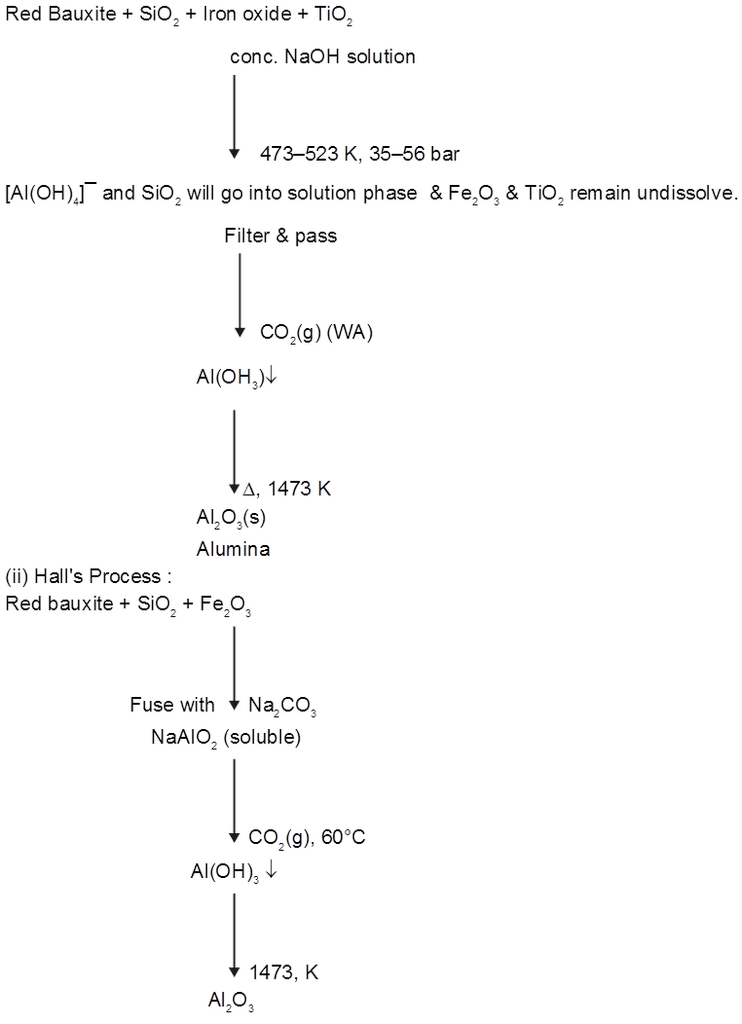
(iii) Serpeck's process :
Used for white bauxite containg silica as impurities.
Al2O3 + N2 + 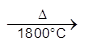 Coke AlN + CO↑
Coke AlN + CO↑
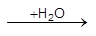 Al(OH)3 + NH3 ↑
Al(OH)3 + NH3 ↑
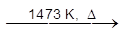 Al2O3 (pure)
Al2O3 (pure)
(2) Electrolytic reduction [Hall-Heroult process] :
Electrolysis of fused Al2O3 is difficult as its MP is very high (2323 K) & It is bed conductor of electricity in fused state.
So Al2O3 is mixed with Na3AlF6 or CaF2. Which lowers the MP (1173 K) & make alumina conducting.
Cathode Þ Al3+ (Melt) + 3e– ® Al(l)
Anode Þ C(s) + O2– (Melt) ® CO(g) + 2e–
C(s) + 2O2– (Melt) ® CO2(g) + 4e–
Overall reaction Þ2Al2O3 + 3C ® 4Al + 3CO2
Note : During electrolytic reduction of aluminum, the carbon anodes are replaced from time to time because oxygen liberated at the carbon anodes react with anode to form CO & CO2.
Electrolytic refining of Al :
Hoop's process : For electrolytic refining of Al, Hoop's process is used.
Anode Þ Impure Al
Cathode Þ Pure Al
Electrolyte Þ Fused salt of Aluminium Fluoride
Note :The fused material remain in three different layer & remain separated because all the layer have different density.
Anode Þ Al(melt) ¾®Al3+ + 3e–
Cathode Þ Al3+ + 3e– ¾®Al
From anode (bottom layer), Al passes into solution as Al3+ ions and then from solution (middle layer) these Al3+ ion pass to cathode (top layer) and get reduced to pure metal.
Extraction of Na : The fused mixture of NaCl and CaCl2 is taken in Down’s cell which consists of circular iron cathode and carbon anode. On passing the electric current the following reactions take place :
Ionisation of NaCl : NaCl  Na+ + Cl–
Na+ + Cl–
Collection of Na at cathode : Na+ + e– ® Na(Reduction).
Collection of Cl2 at anode : Cl– + e– ®Cl (Oxidation), Cl + Cl ® Cl2 .
Na can also be obtained by electrolysing molten NaOH in Castner’s cell.
THERMODYNAMICS OF EXTRACTION : ELLINGHAM DIAGRAM OF A METAL
Ellingham Diagram
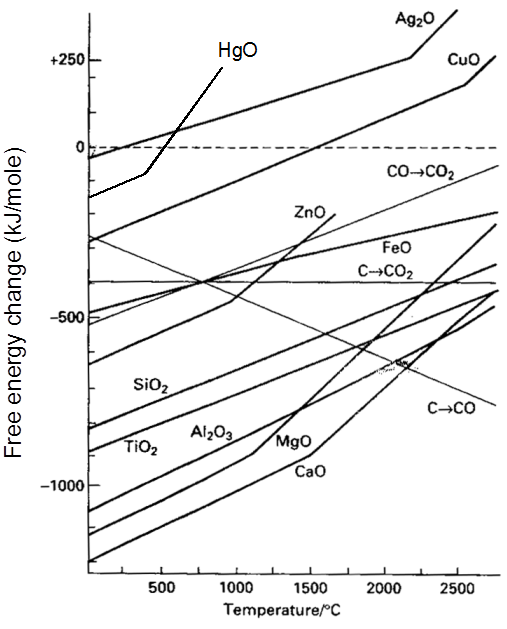
Thermodynamic of extraction :
For a Proces to be spontaneous DG must be negative.
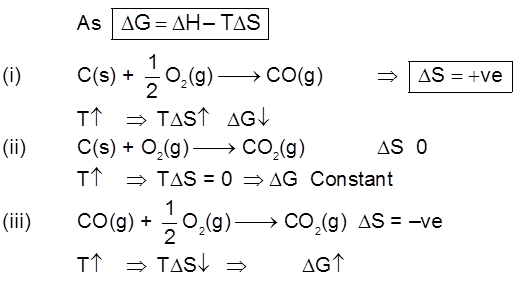
Ellingham Diagram of Metal
XM(s) + 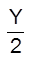 O2(g) ¾® MXOY(s)
O2(g) ¾® MXOY(s)
number of gaseous moles decreae with progress of reaction.
As DS = –ve If T Þ DG
Important Points
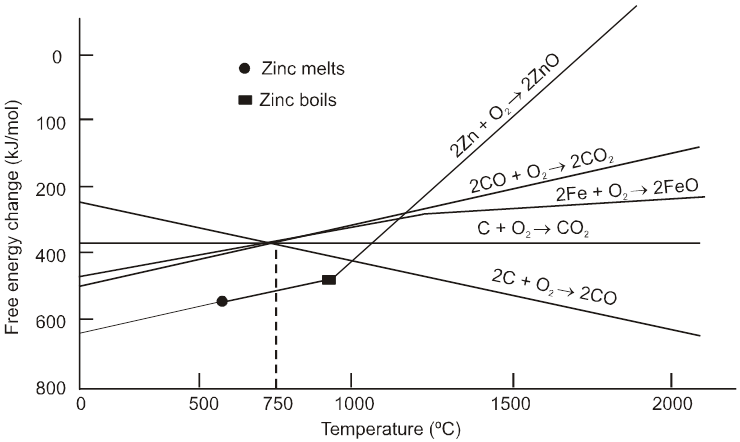
(1) Below 710°C, CO is better reducing agent than C.
(2) All the graphs are upgrowing and on increasing temperature, DG of formation of metal oxide become less negative, while on increasing temperature, DG of C ¾®CO reaction become more negative SO, C can reduce any metal from their metal oxide.
(3) Temperature above which DG of formation of metal oxide become positive, metal oxide become unstable & it decomposes into metal & oxygen.
(4) Theoritically, all metal oxide can be decomposed to give metal & oxygen, if suficiently high temperature is applied.
(5) When plot of one metal oxide is below the others then former metal is capable of reducing the later metal oxide into their metal.
Some important result
(1) All the three oxidation graph of carbon system are above the oxidation graph of ZnO upto temperature 1000°C. At this temperature C is thermodynamically capable of reducing ZnO to Zn. So C is better reducing agent for ZnO to Zn than CO.
(2) Below 1500°C temperature, the graph of MgO lies below the graph of Al2O3, so below this temperature Al cannot reduce MgO, but above this temperature graph of Al2O3 shifts below the graph of MgO so above 1500°C, Al can reduce MgO.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
