- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Factors affecting rate of chemical reaction :
1. Concentration
2. Temperature
3. Nature of reactants & products
4. Catalyst
5. pH of the solution
6. Dielectric constant of the medium.
7. Radiations/light
8. Pressure
9. Electrical & Magnetic field.
The first four factors generally affect rate of almost all reactions while other factors are specific to some reactions only. The common examples of these reactions are :
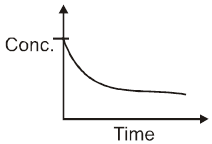
Concentration : We known from law of mass action that Rate is proportional to concentration of reactants. “ So, generally rate of reaction decreases with passage of time, since concentration of reactants decreases.
Temperature :
Nature of reactants & Products :
(a) Physical state of reactants :
Gaseous state > Liquid state > Solid state
Decreasing order of rate of reaction.
(b) Physical size of reactants : As we decreases the particle size rate of reaction increases since surface area increases.
(c) Chemical nature of reactants :
If more bonds are to be broken, the rate of reaction will be slow.
Similarly bond strength is more, rate of reaction will be slow.
Catalyst :
Presence of positive catalyst lower down the activation energy hence increases the rate of reaction.
presence of negative catalyst increases activation energy hence decreases the rate of reaction.
Radiations/light : Radiation are useful for photochemical reaction.
Pressure : Pressure is important factor for gaseous reaction.
Rate Law (Dependence of rate on concentration of reactants) :
The representation of rate of reaction in terms of the concentration of the reactants is called the rate law.
It can only be established by experiments.
Generally rate law expressions are not simple and these may differ for the same reaction on conditions under which the reaction is being carried out.
But for large number of reactions starting with pure reactants we can obtain simple rate laws.
For these reactions :
Rate µ (conc.)order
Rate = K (conc.)order – differential rate equation or rate expression
Where K = Rate constant = specific reaction rate = rate of reaction when concentration is unity
unit of K = (conc)1– order time–1
Note : Value of K is a constant for a given reaction, depends only on temperature
Order of reaction :
Let there be a reaction m1A + m2B ¾® products.
Now, if on the basis of experiment, we find that
R µ [A]P [B]q Where p may or may not be equal to m1 & similarly q may or may not be equal to m2.
p is order of reaction with respect to reactant A and q is order of reaction with respect to reactant B and (p + q) is overall order of the reaction.
Note : Order of a reaction can be ‘zero’ or any whole number, can be a fractional number and it can even be negative with respect to a particular reactant. But oveall order is not found to be negative for any reaction till observed.
Examples showing different values of order of reactions :
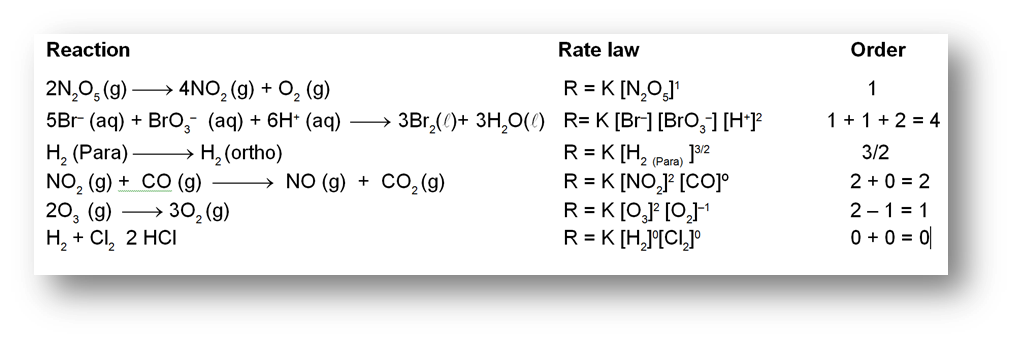
The reaction (2) does not take place in one single step. It is almost impossible for all the 12 molecules of the reactants to be in a state of encounter simultaneously. Such a reaction is called complex reaction and takes places in a sequence of a number of elementary reactions. For an elementary reaction the sum of stoichiometric coefficients = order of the reactions. But for complex reactions order is to be experimentally calculated.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
