- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
General characteristics of catalyst
A catalyst does not initiate the reaction. It simply fastens it.
Only a small amount of catalyst can catalyse the reaction.
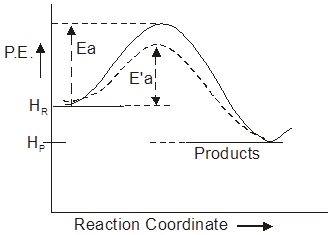
A catalyst does not alter the position of equilibrium i.e. magnitude of equilibrium constant and hence DGº. It simply lowers the time needed to attain equilibrium. This means if a reversible reaction in absence of catalyst completes to go to the extent of 75% till attainment of equilibrium, and this state of equilibrium is attained in 20 minutes then in presence of a catalyst also the reaction will go to 75% of completion before the attainment of equilibrium but the time needed for this will be less than 20 minutes.
A catalyst drives the reaction through a low energy path and hence Ea is less. That is, the function of the catalyst is to lower down the activation energy.
Ea = Energy of activation in absence of catalyst.
E’a = Energy of activation in presence of catalyst.
Ea – E’a = lowering of activation energy by catalyst.
Comparision of rates of reaction in presence and absence of catalyst :
If k and kcat be the rate constant of a reaction at a given temperature T, and Ea and E’a are the activation energies of the reaction in absence and presence of catalyst, respectively, the
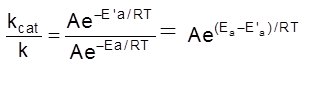
Derivation of a suitable rate law with the help of a suitable mechanism :
Molecularity and Order :
The number of molecules that react in an elementary step is the molecularity of the elementary reaction. Molecularity is defined only for the elementary reactions and not for complex reactions.
No elementary reactions involving more than three molecules are known, because of very low probability of near-simultaneous collision of more than three molecules.
The rate law for the elementary reaction
aA + bB¾® products
rate = k[A]a[B]b, where a + b = 1, 2 or 3.
For an elementary reaction, the orders in the rate law equal the coefficients of the reactants.
While, the order is defined for complex as well as elementary reactions and is always experimentally calculated by the mechanism of the reaction, usually by the slowest step of the mechanism known as rate determining step of the reaction.

Mechanism of a reaction :
Reactions can be divided into
Elementary / simple / single step
Complex / multi-step
ELEMENTARY REACTION :
These reaction take place in single step without formation of any intermediate
For elementary reaction we can define molecularity of the reaction which is equal to no of molecules which make transition state or activated complex because of collisions in proper orientation and with sufficient energy
molecularity will always be a natural no
1 = unimolecular one molecule gets excited (like radioectivity)
2.= bimolecular
3 = trimolecular
Molecularly 3 because the probabilty of simaltaneous collision between 4 or more molecules in proper orientation is very low. For elementary reaction there is only single step and hence it is going to be rate determining step so order of an elementary reaction is its molecularity
Order of elementary reaction w.r.t. reactant = stoichiometric co-efficient of the reactant
H2 + I2 ![]() 2HI ® Simple reaction rate = k [H2] [I2]
2HI ® Simple reaction rate = k [H2] [I2]
2H2 + 2I2 ![]() 4HI (not elementary )
4HI (not elementary )
reaction obtained by multiplying an elementary reaction with some no will not be of elementary nature
H2 + Cl2 ![]() 2HCl order = 0
2HCl order = 0
COMPLEX REACTION :
Reaction which proceed in more than two steps. or having some mechanism. ( sequence of elementary reaction in which any complex reaction procceds)
For complex reaction each step of mechanism will be having its own molecularity but molecularity of net complex reaction will not be defined.
Order of complex reaction can be zero fractions whole no, even negative w.r.t. some species.
Order of reaction or rate law of reaction is calculated with the help of mechanism of the reaction generally using Rate determine step (R.D.S) if given.
Rate law of a reaction is always written in terms of conc. of reactant, products or catalysts but never in terms of conc. of interimediates.
The mechanism of any complex recation is always written in terms of elementary steps, so molecularity of each of these steps will be defined but net molecularity of complex reaction has no meaning.
The mechanism of most of the reaction will be calculated or predicted by using mainly the following approximations.
COMPLICATIONS IN 1ST ORDER REACTION
PARALLEL 1st ORDER REACTION
Frequently a species can react in different ways to give a variety of products. For example, toluene can be nitrated at the ortho, meta, or para positions, We shall consider the simplest case, that of two competing irreversible first-order reactions :
A ![]() B and A
B and A![]() C
C
where the stoichiometric coefficients are taken as unity for simplicity. The rate law is
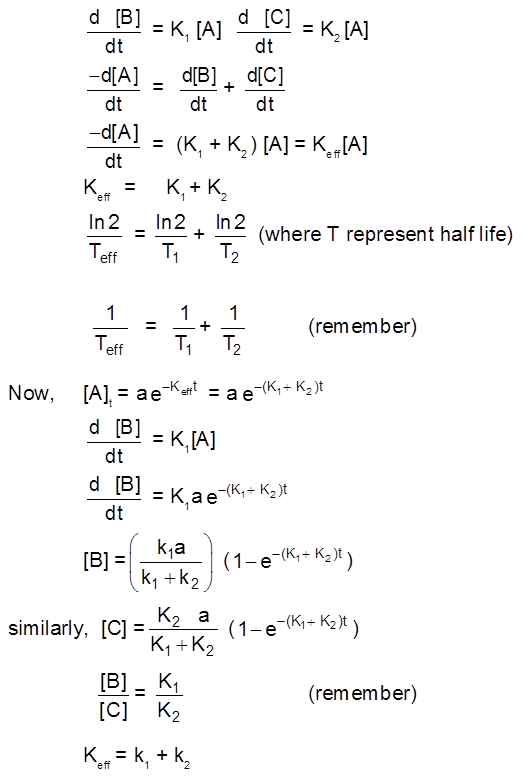

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
