- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Interhalogen compounds
We know that halogen atoms have different electronegativity. Due to this difference in electronegativity the halogen atoms combine with each other and give rise to the formation of binary covalent compounds, which are called interhalogen compounds. These are of four types.
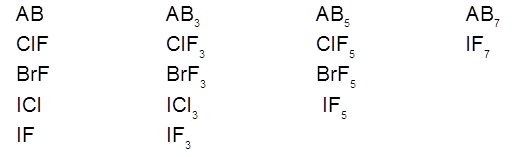
Preparation :
Cl2 + F2 (equal volumes) ![]() 2ClF
2ClF
Cl2 + 3F2 (excess) ![]() 2ClF3
2ClF3
I2 + Cl2 ® 2ICl ;
(equimolar)
Diluted with water :
Br2 (g) + 3F2 ® 2BrF3
F2 is diluted with N2 :
I2 + 3F2 ![]() 2IF3
2IF3
F2 is taken in freon :
Br2 + 5F2 (excess) ® 2BrF5
Properties :
(i) These compounds may be gases, liquids or solids.
Gases : ClF, BrF, ClF3 , IF7
Liquids : BrF3, BrF5
Solids : ICl, IBr, IF3, ICl3.
(ii) All interhalogens are covalent molecules and are diamagnetic in nature since all the valence electrons present as bonding or non-bonding electrons are paired.
(iii) The boiling points increases with the increase in the electronegativity difference between A and B atoms.
(iv) Interhalogen compounds are more reactive than the parent halogens but less reactive than F2.
(v) Hydrolysis : All these undergo hydrolysis giving halide ion derived from the smaller halogen and a hypohalite (when AB), halite (when AB3), halate (when AB5), and perhalate (when AB7) anion derived from the larger halogen.
AB + H2O ® HB + HOA
BrCl + H2O ® HCl + HOBr
ICl + H2O ® HCl + HIO2
Uses :
(i) These compounds can be used as non aqueous solvents.
(ii) Interhalogen compounds are very useful fluorinating agents.
(iii) ClF3 and BrF3 are used for the production of UF6 in the enrichment of 235U.
U(s) + 3 ClF3 (l) ® UF6 (g) + 3 ClF (g)

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
