- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chapter 2:
solutions
1.Introduction : A solution is a homogeneous mixture of two or more substances which are chemically non-reacting. We come across many types of solutions in our daily life. e.g., solid-liquid, liquid-liquid, gas-gas. In this chapter we will learn several properties of solutions and their applications.
Solution: A homogeneous mixture of two or more substances is known as solution
Solute: The substance present in smaller amount in a solution is called solute.
Solvent: The substance present in larger amount in a solution is called solvent.
Types of Solutions
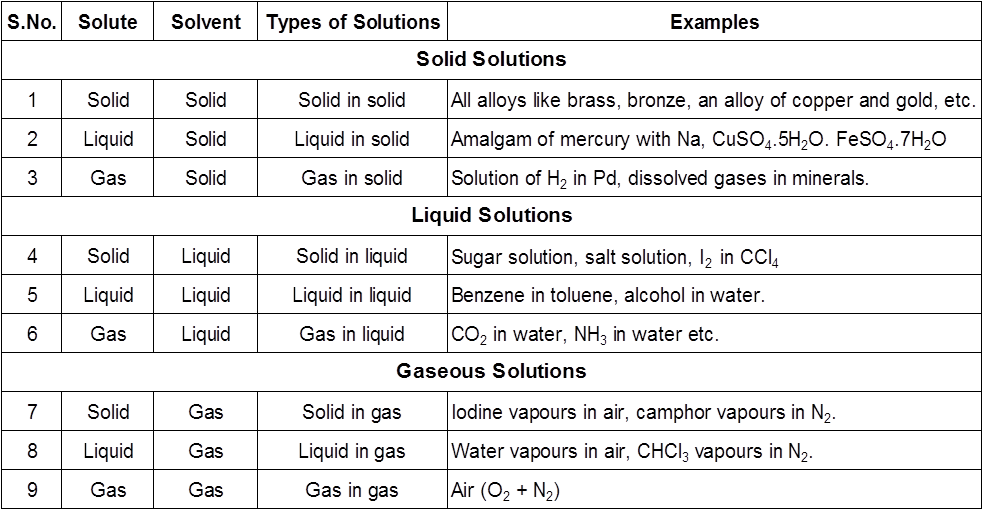
The concentration of a solution can be expressed by different concentration terms which are described as follows.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
