1. Electrochemical cells
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chapter 3
electrochemistry
Introduction
Batteries are everywhere in modern societies. They provide the electric current to start our autombiles and to power a host of products such as pocket caculators, digital watches, heart pacemaker, radio, and tape recorders.
Electrochemistry is the area of chemistry concerned with the interconversion of chemical and electrical.A battery is a an electrochemical cell, a device for interconverting chemical and electrical energy. A battery takes the energy relased by a spontaneous chemical reaction and uses it to produce electricity.
Electrochemical cell
It is device for converting chemical energy in to electrical energy.
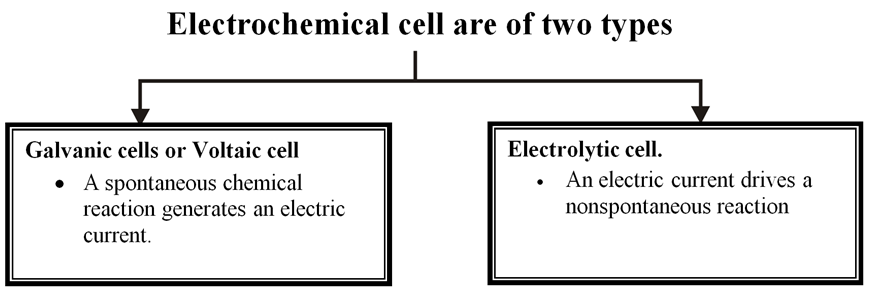
The two types of cells are therefore reverse of each other
Construction/ Working principle
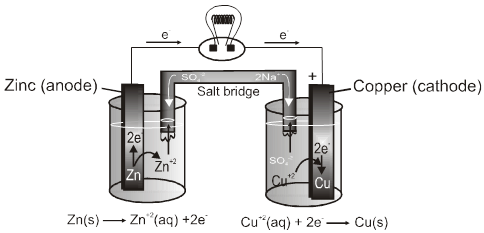
When ever an metal strip is put in an electrolyte the process of oxidation and reduction takes place simultaneously within the system. Due to this there is a potential difference between the metal phase and the liquid phase.
On joining the metal strips through a wire (of negligible resistence) the current flows as long as the potential difference exists between the metal phase and the liquid phase.
I Anode :
Some metals (which are reactive) are found to have tendency to go into the solution phase when these are placed in contact with their ions or their salt solutions.
For example : Zn rod is placed in ZnSO4solution .
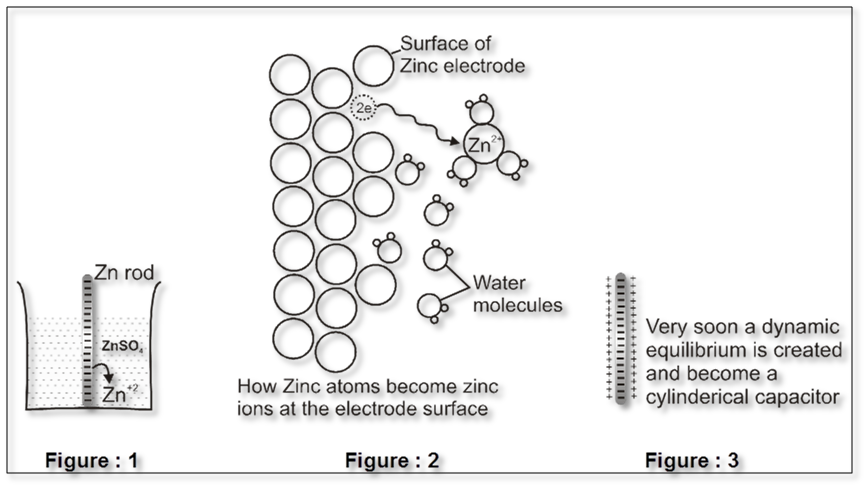
The Zn atom or metal atoms will move in the solution to form Zn+2. After some time following equilibrium will be established.
Zn(s) ![]() Zn2+ +2e–
Zn2+ +2e–
There will be accumulation of sufficient negative charge on the rod which will not allow extra zinc ions to move in the solution. i.e. solution will be saturated with Zn+2 ions.
The positive charge will be more concentrated nearly the rod.
The extra positive charge of the solution will be more concentrated around the negatively charged rod. An electrical double layer is developed in the system and hence a potential difference is created between the rod and the solution which is known as electrode potential
This particular electrode is known as anode :
- On anode oxidation will take place. (release of electron).
- To act as source of electrons.
- It is of negative polarity.
- The electrode potential is represented by EZn(s) / Zn2+ (aq)
II Cathode :
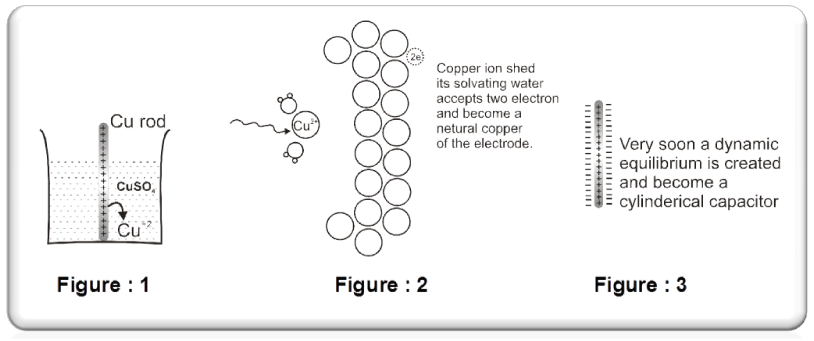
Some metals(Cu, Ag, Au etc.,) are found to have the opposite tendency i.e. when placed in contact with their aqueous ions, the ions from the solution will get deposited on the metal rod.
The following equilibrium will be established :
Cu2+ +2e– Cu(s).
So rod will have deficiency of electron (positive charge).Extra negative charge will surround this positively charged rod and form double layer. An electrical double layer is developed in the system and hence a potential difference is created between the rod and the solution which is known as electrode potential. This will be known as cathode.
- At cathode reduction will take place.(gain of e– will take place)
- To act as sink of electron.
- Positive polarity will be developed.
- Their electrode potential can be represented by : ECu2+(aq)/Cu(s)
Anode :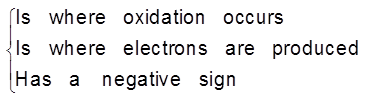
Cathode :
Construction of Cell :
It has two half–cells,each having a beaker containing a metal strip that dips in its aqueous solution.
The metal strips are called electrodes and are connected by an conducting wire.
Two solutions are connected by a salt bridge.
The oxidation and reduction half reactions occur at a separate electrodes and electric current flows through the wire.
Selection of electrolyte for Salt Bridge :
The electrolyte in salt bridge should be such that speed of it's cation equals speed of it's anion in electrical field.
For that charge and sign of the ions should be almost equal.
Transport number of cation = Transport number of anion
or
Mobility of cation = Mobility of anion
KCl is generally preffered but KNO3 or NH4NO3 can also be used.
If Ag+, Hg2+, Pb2+, Tl+ ions are present in a cell then in salt bridge KCl is not used because there can be formation of precipitate of AgCl, Hg2Cl2, PbCl2 or TlCl at mouth of tube which will prevent the migration of ions and its functioning will stop.
Functions of Salt Bridge :
A salt bridge is a U–shaped inverted tube that contains a gel permeated with an inert electrolyte.
It connects the solution of two half cell to complete the circuit.
It minimise the liquid junction potential. The potential difference between the junction of two liquids.
It maintains the electhical neutrality of the solution in order to give continious flow or generation of current.
" The simultaneous electrical neutrality of the anodic oxidation chamber and cathodic reduction chamber is due to same mobility or velocity of K+ and NO3– ions taken into salt bridge.
If the salt bridge is removed then voltage drops to zero.
The ions of the inert electrolyte do not react with other ion in the solution and the ions are not oxidised or reduced at the electrodes.
Generally tube is filled with a paste of agar-agar powder with a natural electrolyte/generally not common to anionic/cathodic compartment with porous plugs at each mouth of tube.
It prevents mechanical mixing of two electrolytic solution.
Electrode Potential :
The driving force that pushes the negative charge electrons away from the anode and pulls them towards the cathode is an electrical potential called electromotive force also known as cell potential or the cell voltage. Its unit is volt
The potential difference devepoled between metal electrode and its ions in solution in known as electrode potential.
Electrode potential depends upon :
- Concentration of the solution.
- Nature of the metal.
- Nature of the electrolyte.
- Pressure temperature coditions.
The potential difference developed between metal electrodes and the solution of its ions at 1 M concentration at 1 bar pressure and 298 K is known as standard electrode potential.
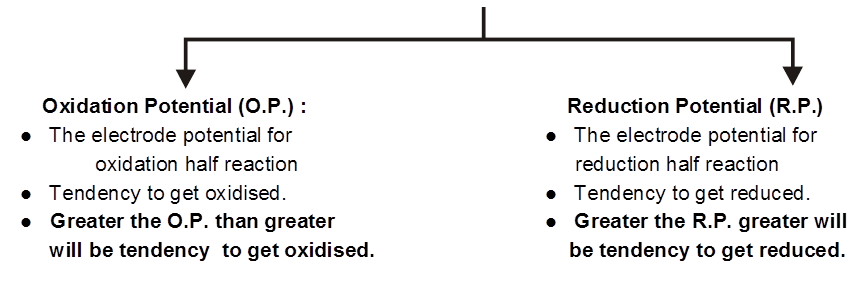
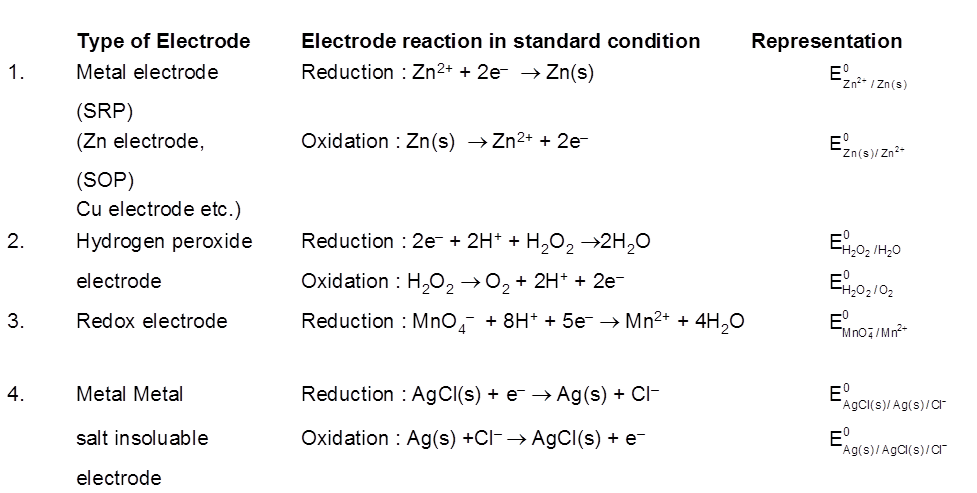
Reference electrode :
The potential of a singal electode cannot be determined what were the potential difference between two electrodes can be accurately measured using a reference electrode.
An electrode is chosen as a reference with respect to which all other electrodes are valued.
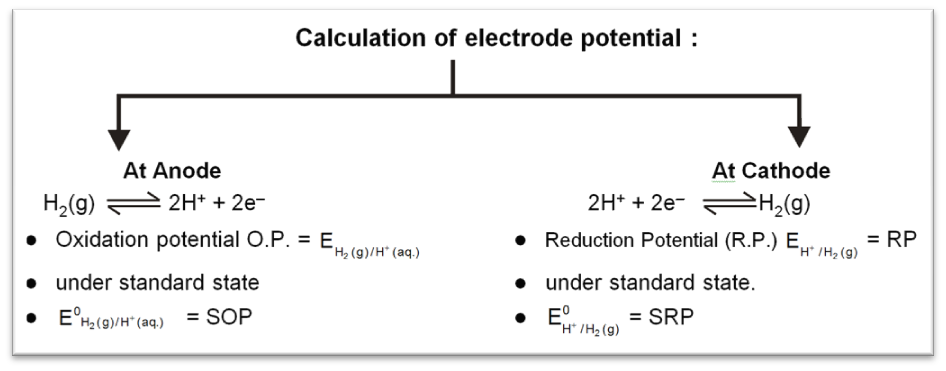
Standard Hydrogen Electrode (SHE) is taken as standard reference electrode. Its electrode potential is arbitrarily assumed to be 0.00 volt.
Standard Hydrogen Electrode (SHE) consists of a platinum electrode in contact with H2 gas and aqueous H+ ions at standard state conditions (1 atm H2 gas, 1 M H+ (aq), 25°C).
2H+ (aq, 1M) + 2e– → H2 (g, 1 atm) [E° = 0V]
H2(g, 1atm) → 2H+ (aq, 1M) + 2e– [E° = 0V]
Cell potential :
The difference in electrode potentials of the two half cell reactions (oxidation half cell and reduction half cell) is known as emf of the cell or cell potential.
The emf of the cell or cell potential can be calculated from the values of electrode potential of the two half cell constituning the cell. The following three methode are in use :
When oxidation potential of anode and reduction potential of cathode are taken into account :
E°cell = oxidation potential of anode + reduction potential of cathode
E°ox (anode) + E°red(cathode)
When reduction potential of both electrodes are taken into account :
E°cell = Reduction potential of cathode – Reduction potential of anode
= E°cathode – E°anode C both are reduction potential.
When oxidation potential of both electrodes are taken into account :
E°cell = oxidation potential of anode – Oxidation potential of cathode
= E°ox (anode) – E°ox (cathode)
The standard cell potential E° is the cell potential when both reactants and products are in their standard states – solutes at 1 M concentration, gases at a potential pressure of 1 atm, solids and liquids in pure from, with all at a specified temperature, usually 25° C.
E°cell is intensive property so on multiplying/Dividing cell reaction reaction by any number, the E°cell value would not change.
Free energy changes for cell reaction :
The free energy changeDG (a thermochemical quantity) and the cell potential E(an electrochemical quantity) both measure the driving force of a chemical reaction.
The values ofDG and E are directly proportional and are related by the equation.
DG = –nFE
where
n = Number of moles of electron transfered in the reaction.
F = Faraday constant = 96485 C/mole e– 96500 C/mole e–
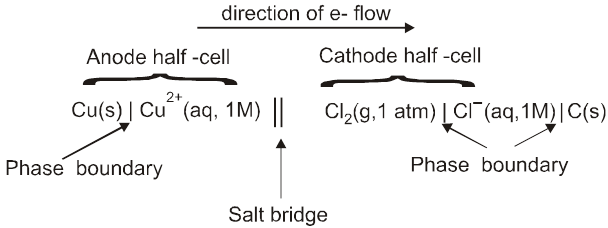
Shorthand Notation for Galvanic Cells
We require two half cells to produce an electrochemical cell, which can be represented by follwing few rules;
The anode half-cell is always written on the left followed on the right by cathode half cell.
The separation of two phases (state of matter) is shown by a vertical line.
The various materials present in the same phase are shown together using commas.
The salt bridge is represented by a double slash (||).
The significant features of the substance viz. pressure of a gas, concentration of ions etc. are indicated in brackets immediately after writing the substance.
For a gas electrode, the gas is indicated after the electrode for anode and before the electrode in case of cathode. (i.e Pt H2 / H+ or H+ /H2 Pt)
For SHE reference potential is taken to be zero at all temperature.
SOP = – SRP = 0 for SHE.
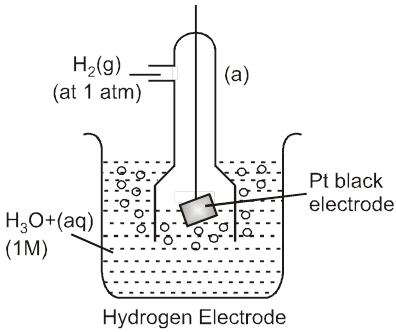
To calculate standard potential of any other electrode a cell is coupled with standard hydrogen electrode (SHE) and it's potential is measured that gives the value of electrode potential of that electrode.
Anode : Zinc electrode
Cathode : SHE
Cell : Zinc electrode || SHE
Cell potential :
Ecell = ![]() – E°Zn2+/Zn
– E°Zn2+/Zn
= 0.76 V (at 298 K experimentaly)
So, E0Zn2+/Zn = – 0.76 V (SRP)
E0 Zn/Zn2+(aq) = 0.76 V(SOP)
So, w.r.t. H2, Zn has greater
tendency to get oxidised. In similar manner reduction potentials (SRP) at 298 K for many other electrodes are calculated and are arranged in a series increasing order known as electro chemical series.
Electrochemical Series :

Calculation of Electrode Potential of unknown electrode with the help of given (two) electrode.
Obtain the reaction of the 3rd electrode with the help of some algebraic operations on reactions of the given electrodes.
Then calculate DG of the 3rd reaction with the help of some algebaric operations of DG0 of 1st and 2nd reactions.
Use DG0 = –nF E0elec. to calculate unknown E.P.
![]() is intensive property so if we multiply/Devide electrode reaction by any number the
is intensive property so if we multiply/Devide electrode reaction by any number the ![]() value would not changed
value would not changed
i.e. Zn2+ + 2e– ® Zn(s) [E° = – 0.76 V[
Multiply by 2
2Zn2+ + 4e– ® 2Zn(s) [E° = – 0.76 V (remain same)]
2. Nernst equation
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Nernst Equation
Cell potentials depend on temperature and on the composition of the reaction mixtures.
It depends upon the concentration of the solute and the partial pressure of the gas, if any.
The dependence upon the concentration can be derived from thermodynamics.
From thermodynamics
DG = DG° + RT ln Q
– nFE = – nFE° + 2.303 R T log Q
E = E° – ![]() log Q
log Q
Take
T = 298 K ,
R = 8.314 J/mol K ,
F = 96500 C
Now we get,
E = E° – ![]() log Q
log Q
Where
n = number of transfered electron ,
Q = reaction quotient
Nernst equation can be used to calculate cell potentials for non standard conditions also.
Nernst equations can be applied to half cell reactions also.
Applications of Nerst equation
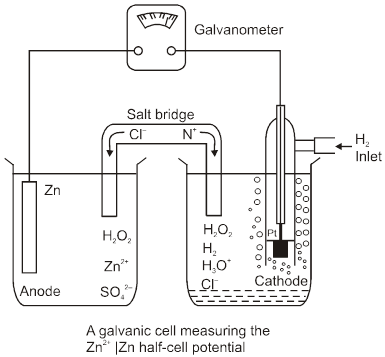
Nernst Equation for Electrode Potential
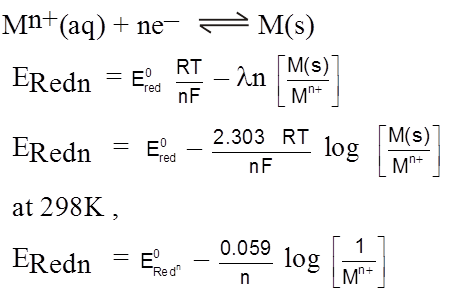
Hydrogen Electrode
H2(g) ![]() 2H+(aq) + 2e–
2H+(aq) + 2e–
E = E0 –![]() log
log 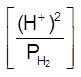
Metal–metal soluble salt electrode.
Zn2+ + 2e–  Zn(s)
Zn(s)
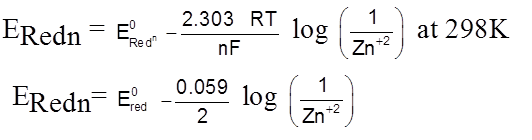
Gas – electrode Hydrogen electrode.
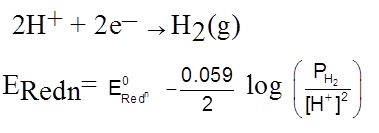
Redox electrode
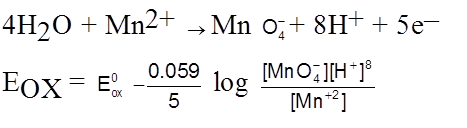
Nernst Equation for cell Potential :
aA + bB ![]() CC + dD
CC + dD
Ecell = ![]() –
– ![]() lnQ
lnQ
n – no. of electrons which gets cancelled out while making cell reaction.
Equilibrium in electrochemical cell
G0 = – nF Eºcell
G = – nF Ecell
From thermo dynamics
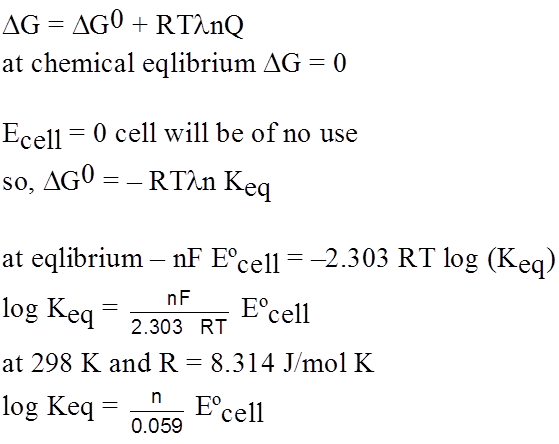
Concentration cells :
A concentration cell consists of two electrodes of the same material, each electrode dipping in a solution of its own ions and the solution being at different concentrations.
The two solutions are separated by a salt bridge.
e.g. Ag(s) | Ag+ (a1) || Ag+ (a2) | Ag(s) (a1 < a2) a1 , a2 are concentrations of each half cell
At LHS electrode Anode : Ag (s) ![]() Ag+(a1) + e–
Ag+(a1) + e–
At RHS electrode Cathode : Ag+(a2) + e– ![]() Ag(s)
Ag(s)
The net cell reaction is : Ag+ (a2) ![]() Ag+ (a1)
Ag+ (a1)
The nernst eq. is
Ecell = – ![]() log
log ![]() (Here n = 1, Temp, 298 K)
(Here n = 1, Temp, 298 K)
Likewise, the e.m.f. of the cell consisting of two hydrogen electrodes operating at different pressure P1 and P2 (P1 > P2 ) and dipping into a solution HCl is :
Ecell = ![]() log
log ![]() (at 298 K)
(at 298 K)
Solubility product and EMF (Metal Metal Insoluble Salt Electrode) :
A half cell containing metal M and its sparingly soluble salt MA in a saturated solution.
i.e M(s) | MA (satd) or a metal, its sparingly soluble salt in contact with a solution of a soluble salt NaA of the same anion, i.e. M(s) | MA(s) | NaA is set up.
The solubility product of a sparingly doubles salt is a kind of equilibrium constant.
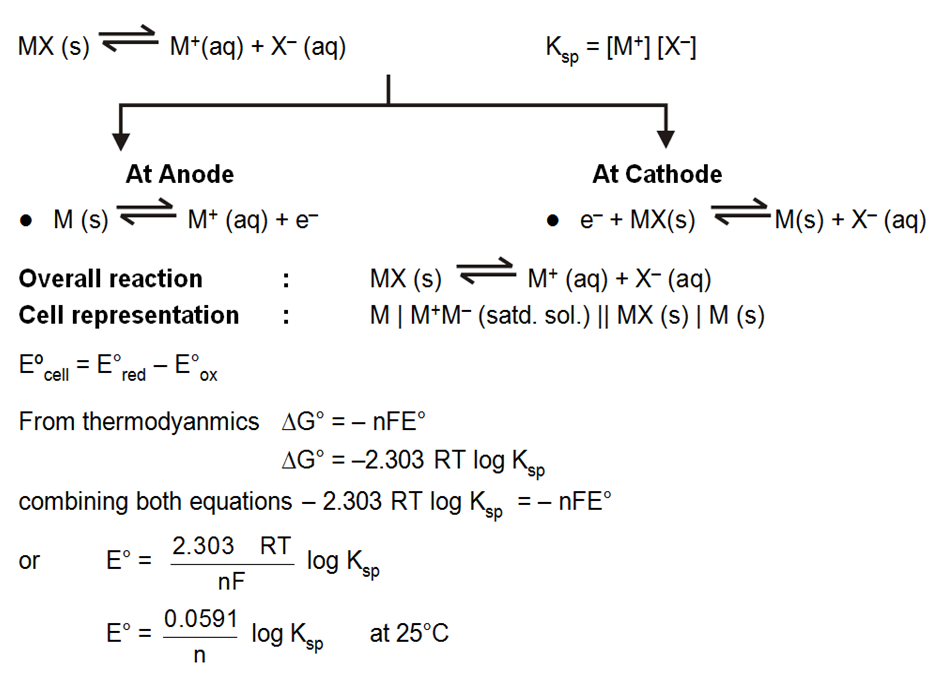
Work done by a cell :
(i) Let 'n' faraday charge be taken out of a cell of EMF 'E' ; then work done by the cell will be calculated as : work = Charge × Potential = nFE
(ii) Work done by cell = Decrease in free energy
so –DG = nFE
or Wmax = + nFEº where Eº is standard EMF of the cell
3. Conductance of electrolytic solutions
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Conductance of electrolytic solutions
Electrolytic Conductance :

Factors Affecting Conductance & Resistance :
1. Solute – Solute interactions (Inter – Ionic force of attraction) Greater the force of attraction, greater will be the resistance.
Force ![]() Charge
Charge
2.Solute – Solvent Interaction (Hydration/Solvation of Ions) Greater the solvation
Solvation ![]() Charge
Charge ![]() greater will be resistance Li+ (Hydrated largest) Cs+ (Hydrated smallest)
greater will be resistance Li+ (Hydrated largest) Cs+ (Hydrated smallest)
resistance of LiCl > resistance of CsCl
3. Solvent solvent interaction (Viscosity) : greater the viscosity greater will be resistance
4. Temperature
T ![]() R
R ![]()
5. Nature of electrolyte
Weak electrolyte – high resistance strong electrolyte – Low resistance
Resistance :
R = ![]() (Ohm's law (
(Ohm's law ( ![]() )
)
R = ![]()
![]() – resistivity/specific resistance
– resistivity/specific resistance
– resistance of unit length wire of unit area of cross section = constant = (![]() m)
m)
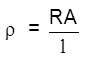
Resistivity of a solution is defined as the resistance of the solution between two electrodes of 1 cm2 area of cross section and 1 cm apart.
or
Resistance of 1 cm3 of solution will be it's resistivity.
Conductance :

= S (Siemens)
Conductivity/specific conductance
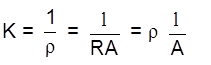
unit –1 cm–1
= conductivity of 1 cm3 of solution
a concentration of ions
K = ![]() G =
G = ![]()
K ![]() ( no. of ions) no. of charge carries
( no. of ions) no. of charge carries
Since conductivity or resistivity of the solution is dependent on it's concentration, so two more type of conductivities are defined for the solution.
Molar conductivity/molar conductance ((lm) :
Conductance of a solution containing 1 mole of an electrolyte between 2 electrodes which are 1cm apart.
Let the molarity of the solution 'C'
C moles of electrolyte are present in 1 Lt. of solution.
so molar conductance = K
![]()
Its units are Ohm–1 cm2 mol–1
Equivalent conductance : Conductivity of a solution containing 1 g equivalent of the electrolyte.
leq – equivalent conductivity/conduction.
leq = 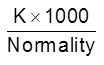
Its units are Ohm–1 cm2 eq–1
Variation of conductivity and molar conductivity with concentration
Conductivity always decreases with the decrease in concentration both for weak and strong electrolytes.
The number of ions per unit volume that carry the current in a solution decreases on dilution.
Molar conductivity increases with decreases in concentration. This is because the total volume, V of solution containing one mole of electrolyte also increases.
Molar conductivity is the conductance of solution.
When concentration approaches zero, the molar conductivity is known as limiting molar conductivity and is represented by the symbol ![]() .
.
Strong Electrolytes :
For strong electrolytes. ![]() increses slowly with dilution and can be represented by the equation
increses slowly with dilution and can be represented by the equation
![]()
The value of the constant 'A' for a given slovent and temperature depends on the type of electrolyte i.e. the charges on the cations and anion produced on the dissociation of the electrolyte in the solution.
Example : Thus NaCl, CaCl2, MgSO4 are known as 1-1 , 2-1 and 2-2 electrolyte respectively.
All electrolytes of a particular type have the same value for 'A'.
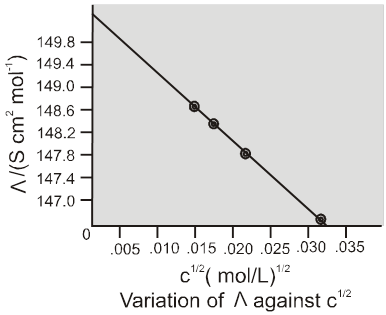
Weak electrolytes
Weak electrolytes like acetic acid have lower degree of dissociation at higher concentration and hence for such electrolytes, the change in  with dilution is due to increases in the number of ions in total volume of solution that contains 1 mol of electrolyte.
with dilution is due to increases in the number of ions in total volume of solution that contains 1 mol of electrolyte.
At infinite dilution (i.e. concentration c ![]() zero) electrolyte dissociates completely (a= 1),but at such low concentration the conductivity of the solution is so low that it connot be measured accurately.
zero) electrolyte dissociates completely (a= 1),but at such low concentration the conductivity of the solution is so low that it connot be measured accurately.
Molar conductivity versus c1/2 for acetic acid (weak electrolyte) and potassium chloride
(strong electrolyte in aqueous solutions.
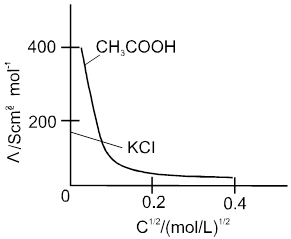
Kohlarausch's Law :
"At infinite dilution, when dissociation is complete, each ion makes a definite contribution towards equivalent conductance of the electrolyte irrespective of the nature of the ion with which it is associated and the value of equivalent conductance at infinite dilution for any electrolyte is the sum of contribution of its constituent ions."
i.e., l¥ = l+ + l–
At infinite dilution or near zero concentration when dissociation is 100%, each ion makes a definite contribution towards molar conductivity of electrolyte irrespective of the nature of the other ion. (because interionic forces of attraction are zero)
![]()
V+= no. of cation in one formula unit of electrolyte, = no. of anions in one formula unit of electrolyte
For NaCl = 1 v- = 1
For Al2(SO4)3 = 2 v- = 3
![]()
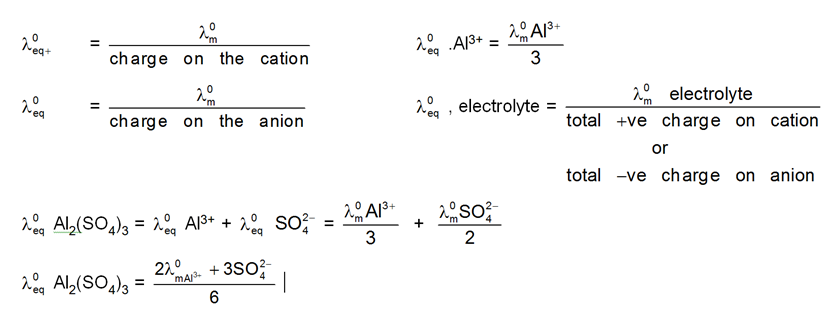
Applications of Kohlaraushch's law
Calculate ![]() for any electrolyte from the
for any electrolyte from the ![]() of individual ions.
of individual ions.
etermine the value of its dissociation constant once we known the ![]() and
and ![]() at a given concentration c.
at a given concentration c.
Degree of dissociation : At greater dilution the ionization become 100%, therefore called infinite dilution.
At lower dilution the ionization (dissociation into ions) is less than 100% and equivalent conductance become lower,
i.e., leq < l°eq
degree of dissociation
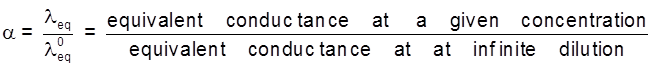
Dissociation constant of weak electrolyte :
KC = ![]()
a = degree of dissociation
C = concentration
The degree of dissociation then it can be approximated to the ratio of molar conductivity ![]() at the concentration c to limiting molar conductivity,
at the concentration c to limiting molar conductivity, ![]() , Thus we have :
, Thus we have :
![]()
But we known that for a weak electrolyte like acetic acid.
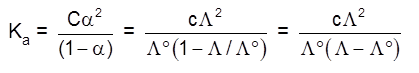
Solubility(s) and KSP of any sparingly soluble salt.
Sparingly soluble salt = Very small solubility
Solubility = molarity = 0
so, solution can be considered to be of zero conc or infinite dilution.
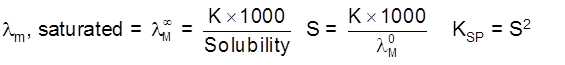
Variation of K, lm & leq of solutions with Dilution
Kconc. of ions in the solution. In case of both strong and weak electrolytes on dilution the concentration of ions will decrease hence K will decrease.
4. Electrolytic cells and electrolysis
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Electrolytic cells and electrolysis
Electrolysis :
Electrolyte is a combination of cations and anions which in fused state can conduct electricity.
This is possible due to the movement of ions from which it is made of and electrolyte.
The process of using an electric current to bring about chemical change is called electrolysis.
Electrolysis is a process of oxidation and readuction due to current in the electrolyte.
The product obtained during electrolysis depends on following factors.
The nature of the electrolyte
The concentration of electrolyte
The charge density flowing during electrolysis.
The nature of the electrode
Active vs Inactive electrodes :
The electrodes in the cell that are active because the metals themselves are components of the half reactions.
As the cell operates, the mass of the zinc electrode gradually decreases, and the [Zn2+] in the anode half – cell increases. At the same time, the mass of the copper electrode increases and the [Cu2+] in the cathode half – cell decreases; we say that the Cu2+ "plates out" on the electrode.
For many redox reactions, however, there are no reactants or products capable of serving as electrodes. Inactive electrodes are used, most commonly rods of graphite or platinum, materials that conduct electrons into or out of the cell but cannot take part in the half -reactions.
In a voltaic cell based on the following half reactions, for instance, the species cannot act as electrodes
2I–(aq) ![]() I2(s) +2e– [anode ; oxidation]
I2(s) +2e– [anode ; oxidation]
MnO4– (aq) + 8H+ (aq) + 5e– ![]() Mn2+ (aq) + 4H2O(λ) [cathode ; reduction]
Mn2+ (aq) + 4H2O(λ) [cathode ; reduction]
Therefore, each half – cell consists of inactive electrodes immersed in an electrolyte solution that contains all the species involved in that half -reaction. In the anode half-cell, I– ions are oxidized to solid I2. The electrons released flow into the graphite anode, through the wire, and into the graphite cathode. From there, the electrons are consumed by MnO4– ions as they are reduced to Mn2+ ions.
Examples of Electrolysis
Using inert (pt/graphite) electrodes.
Cathode (red) : Pb2+ + 2e– ![]() Pb(s) E0 = 0.126V
Pb(s) E0 = 0.126V
Anode : 2Br- ![]() Br2 + 2e- E0 = – 1.08 V
Br2 + 2e- E0 = – 1.08 V
Ecell = – 0.126 – (0.108) x 10 = – 1.206 V
Eext > 1.206 V
Electrolysis of CuSO4 molten
Cathode : Cu2+ + 2e– ![]() Cu [E0 = +0.34 V]
Cu [E0 = +0.34 V]
Anode : ![]() [E0 = – 2.05 V]
[E0 = – 2.05 V]
H2S2O8 – marchall's acid peroxy disulphuric acid.
Ecell = 0.34 – (2.05) = – 1.71 V (negative not feasible)
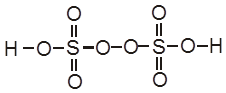
Electrolysis of aq CuSO4
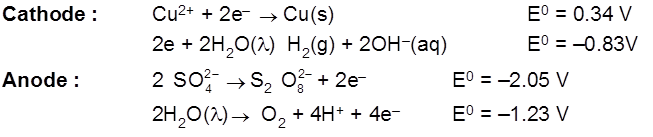
Electrolysis of aq NaBr solution (initially PH = 7)
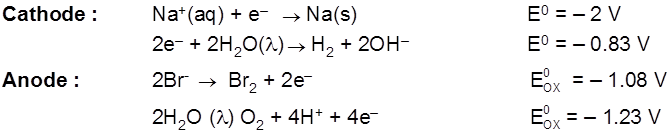
Electrolysis of aq NaCl
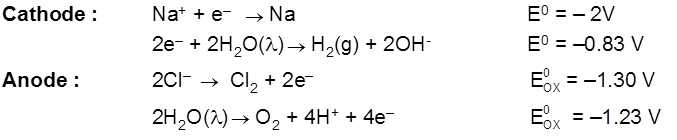
Rate of production of Cl2 is more than rate of production of O2 gas.
Note : According to thermodynamics, oxidation of H2O to produce O2 should take place on anode but experimentally (experiment from chemical kinetics) the rate of oxidation of water is found to be very slow. To increase it's rate, the greater potential difference is applied called over voltage or over potential but because of this oxidation of Cl– ions also become feasible and this takes place on anode.

Electrolysis using attackable (reactive) electrodes.
- Electrolysis of aq. CuSO4 using Cu electrode.
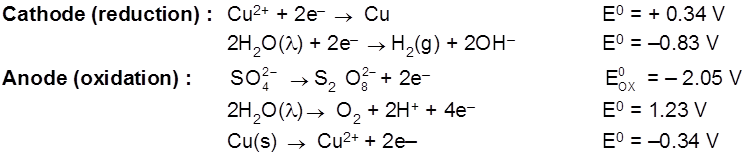
electrolytic refining
- AgNO3(aq) using Cu cathode & Ag anode.
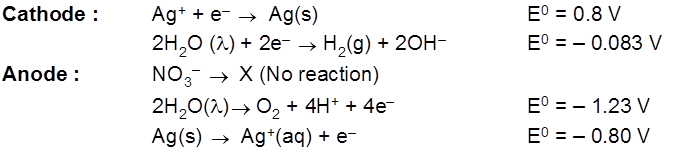
Faraday's Law of Electrolysis :
1st Law : The mass deposited/released/produced of any substance during electrolysis is proportional to the amount of charge passed into the electrolyte.
WQ
W = ZQ
Z – electrochemical equivalent of the substance.
Unit of Z =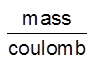 = Kg/C or g/C
= Kg/C or g/C
Z = Mass deposited when 1 C of charge is passsed into the solution.
Equivalent mass (E) : mass of any substance produced when 1 mole of e– are passed through the solution during electrolysis.
E = 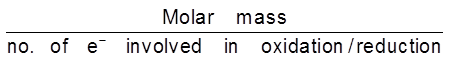
e.g.
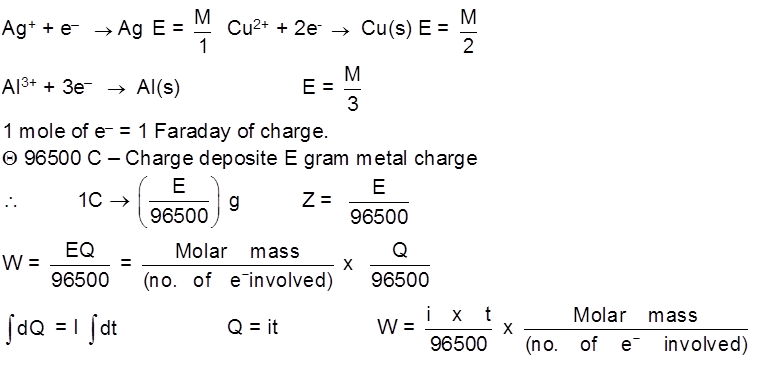
2nd Law : When equal charge is passed through 2 electrolytic cells and this cells are connected in series then mass deposited at electrode will be in the ratio of their electrochemical equivalents or in the ratio of their equivalent masses. 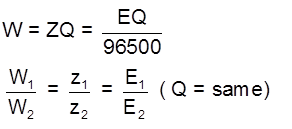
Current Efficiency :
current efficiency = ![]() x 100
x 100
current efficiency = 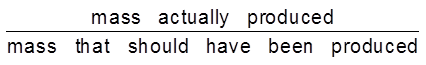 x 100
x 100
5. Batteries
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Batteries
Battery :
A battery is a an electrochemical cell, a device for interconverting chemical and electrical energy. A battery takes the energy relased by a spontaneous chemical reaction and uses it to produce electricity.
Primary Batteries : In the primary batteries, the reaction occurs only once and after use over a period of time battery becomes dead and cannot be reused again. The most familiar example of this type is the dry cell (known as Leclanche cell after its discoverer) which is used commonly in our transistors and clocks. All substances used are either pure solids or pure liquids.
Types of primary batteries :
(i) Dry cell %
Anode : Zn(s)
Cathode : MnO2(s)
Electrolyte : Paste of NH4Cl + ZnCl2 in starch
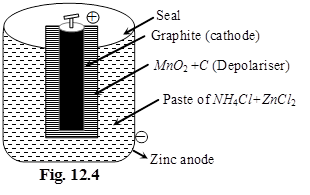
Cathode :
MnO2 + NH4+ + e– ![]() MnO(OH) + NH3
MnO(OH) + NH3
(Oxidation state of Mn changes from +4 to +3)
Anode :
Zn ![]() Zn2+ + 2e–
Zn2+ + 2e–
(ii) Mercury cell :
Anode: HgO(s)
Cathode : Zn(Hg)
Electrolyte : Paste of KOH + ZnO
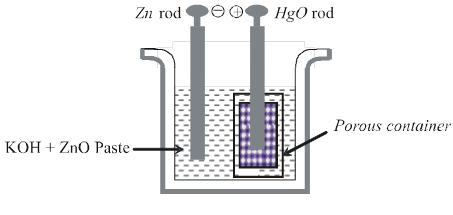
Cathode :
Zn(Hg) + 2OH– ![]() ZnO(s) + H2O(l) + 2e–
ZnO(s) + H2O(l) + 2e–
(amalgam)
Anode :
HgO(s) + H2O(l) + 2e– ![]() Hg(l) + 2OH–
Hg(l) + 2OH–
Secondary Batteries : A secondary cell afer use can be recharged by passing current through it in the opposite direction so that it can be used again. A good secondary cell can udnergo a large number of discharing and charging cycles. The most important secondary cell is the lead storage battery commonly used in automobiles and inverters.
Anode : Pb(s)
Cathode : PbO2(s)
Electrolyte : 38 % Conc. H2SO4 solution Ecell = 2.05 V.
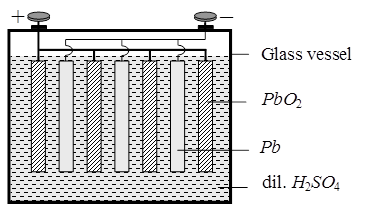

During the working of the cell discharge H2SO4 will be consumed so it's concentration in the solution hence density of the solution will decrease during charging of the cell PbSO4 will get converted into Pb(s) and, PbO2(s) and H2SO4 will be produced.
Nickel – cadmium battery.
Ecell = constant as cell reaction has pure solide/liquids only.
Anode : Cd(s)
Cathode : NiO2(s)
Electrolyte : KOH
Cd + 2OH– ![]() Cd(OH)2 + 2e–
Cd(OH)2 + 2e–
2e– + NiO2 + 2H2O ![]() Ni(OH)2(s) + 2OH–
Ni(OH)2(s) + 2OH–
Cd(s) + NiO2(s) + 2H2O(λ)![]() Cd(OH)2(s) + Ni(OH)2(s)
Cd(OH)2(s) + Ni(OH)2(s)
6. Fuel cells
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Fuel cell
Fuel cells (H2 – O2 cell) : Galvanic cells those are designed to convert the energy of combustion of fuels like hydrogen, methane, methanol etc. directly into electrical energy are called fuel cells.
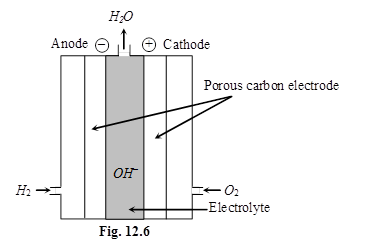
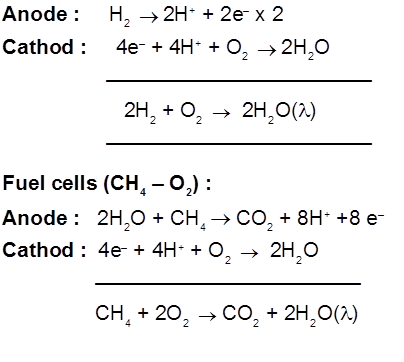
7. Corrosion
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Corrosion
It is a process of deterioration of a metal as a result of its reaction with air or water (environment) surrounding it.
In case of iron, corrosion is called rusting. Chemically, rust is hydrated form of ferric oxide, Fe2O3 .xH2O Rusting of iron is generally caused by moisture, carbon dioxide and oxygen present in air.
Mechanism of corrosion :
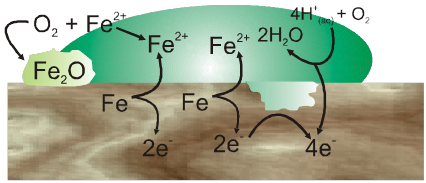
Oxidation :
Fe(s) ![]() Fe2+ (aq) + 2e–
Fe2+ (aq) + 2e–
Reduction :
2O2–(g) + 4H+ (aq) ![]() 2H2O(I)
2H2O(I)
Atomospheric oxidation :
2Fe2+(aq) + 2H2O(l) + 1/2O2 ![]() Fe2O3(s) + 4H+(aq)
Fe2O3(s) + 4H+(aq)
Factors which affect corrosion : The main factors which affect corrosion are
Rate of corrosion µ Rectivity of metal
µ Presence of impurities.
µ Presence of electrolytes.
µ temperature (with in a reason able limit)
Corrosion protection : Corrosion of metals can be prevented in many ways. Some commonly used methods are
(i) By surface coating
(a) By applying, oil, grease, paint or varnish on the surface.
(b) By sacrifical protection; coating/depositing a thin layer of any other metal which does not corrode. For example, iron surface can be protected from corrosion by depositing a thin layer of zinc, nickel or chromium on it.
(c) By Galvanization : Prevention of corrosion of iron by Zn coating.
(ii) cathodic protection : (By connecting metal to a more electropositive metal) In presence of the more electropositive metal, the given metal does not get corroded. For example, iron can be protected from corrosion by connecting it to a block/plate of zinc or magnesium.
(iii) By forming insoluble phosphate or chromate coating : Metal surfaces are treated with phosphoric acid to form an insoluble phosphate. Formation of a thin chromate layer also prevents the corrosion of metals.
(iv) Using anti – rust solutions : Solutions of alkaline phosphates and alkaline chromates are generally used as anti – rust solutions.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
