- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Colligative properties & constitutional properties :
Constitutional Properties : Properties which are dependent on nature of particles are constitutional properties like electrical conductance.
Colligative properties : The properties of the solution which are dependent only on the total no. of particles relative to solvent/solution or total concentration of particles in the solution and are not dependent on the nature of particle i.e. shape, size, neutral /charge etc. of the particles.
There are 4 colligative properties of solution :
Osmotic pressure
Relative lowering in vapour pressure ![]()
Elevation in b.p. (DTb)
Depression in freezing pt. (DTf)
(i) Osmosis & Osmotic pressure :
Osmosis: The spontaneous flow of solvent particles from solvent side to solution side or from solution of low concentration side to solution of high concentration side through a semipermeable membrane (SPM) is known as osmosis.
Ex.
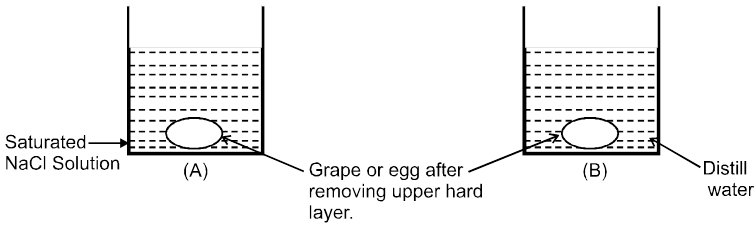
Conclusion : After some time in (A) grape or egg will shrink and in (B) grape or egg will swell.
e.g. (i) A raw mango placed in concentrated salt solution loses water & shrivel into pickle.
(ii) People taking lot of salt, experience water retention in tissue cells. This results in puffiness or swelling called edema.
Biological Importance of Osmosis
The cell walls of plants and animals are semipermeable in nature and thus are responsible for the osmosis. When a cell comes in contact with water a tendency of flow of water into the cell is developed. The pressure developed inside the cell due to the inflow of water is called turgor.
If the cell comes in contact with a concentrated solution, cell would shrink, which is called plasmolysis. Cell walls are able to permit the flow of selected ions and molecules also along with water.
Various processes taking place due to osmosis are :
(a) Absorption of water from soil through the cell walls of roots.
(b) Movement of water from roots to the upper parts of plants & trees.
(c) Various types of locomotion in plants like stretching of leaves, opening of flowers, etc., are also based on osmosis.
(d) Germination is also caused due to osmosis as water moves into the seeds through cell walls.
The phenomenon of osmosis : A solution inside the bulb is separated from pure solvent in the beaker by a semipermeable membrane, Net passage of solvent from the beaker through the memberane occurs, and the liquid in the tube rises untill equilibrium is reached. At equilibrium, the osmotic pressure exerted by the column liquid in the tube is sufficient to prevent further net passage of solvent.
Although the passage of solvent through the membrane takes place in both direction, passage from the pure solvent side to the solution side is more favoured and occurs faster. As a result, the amount of liquid on the pure solvent side decreases, the amount of liquid on the solution side increases, and the concentration of the solution decreases.
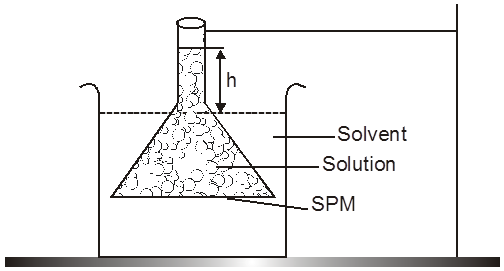
Osmotic Pressure : The equilibrium hydrostatic pressure developed by solution column when it is seperated from solvent by semipermeable membrane is called O.P. of the solution.
p = rgh ;
r = density of soln.
g = acceleration due to gravity;
h = eq. height
1 atm = 1.013 x 105 N/m2
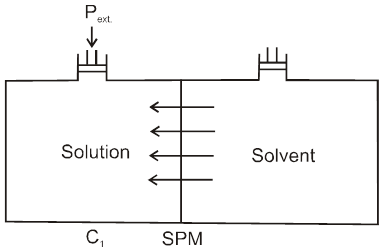
Definition : The external pressure which must be applied on solution side to stop the process of osmosis is called osmotic pressure of the solution.
If two solutions of concentration C1 and C2 are kept separated by SPM, and C1 > C2 then particle movement take place from lower to higher concentration. So, extra pressure is applied on higher concentration side to stop osmosis. And
Pext. = (p1 – p2)
Reverse Osmosis : If the pressure applied on the solution side is more than osmotic pressure of the solution then the solvent particles will move from solution to solvent side. This process is known as reverse osmosis.
Berkely : Hartely device/method uses the above pressure to measure osmotic pressure.
e.g. used in desalination of sea-water.
Vant – Hoff Formula (For calculation of osmotic pressure)
p a concentration (molarity)
a T
p = CST
S = ideal solution constant p = atm. 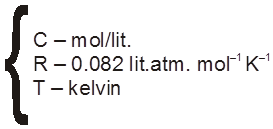
= 8.314 J mol–1 K–1 (exp value)
= R (ideal gas) constant
p = CRT = ![]() RT (just like ideal gas equation)
RT (just like ideal gas equation)
In ideal solution solute particles can be assumed to be moving randomly without any interactions.
 C = total concentration of all types of particles.
C = total concentration of all types of particles.
= C1 + C2 + C3 + s.................
= ![]()
Type of solutions :
(a) Isotonic solution : Two solutions having same osmotic pressure are consider as isotonic solution.
p1 = p2 (at same temperature)
(b) Hypotonic & Hypertonic solutions : If two solutions 1 and 2 are such that p2 > p1 , then 2 is called hypertonic solution and 1 is called hypotonic solution.
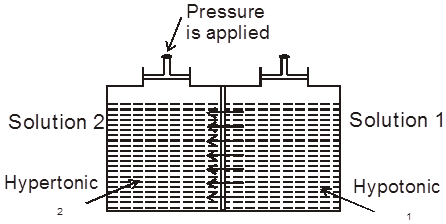
Conclusion :Pressure is applied on the hypertonic solution to stop the flow of solvent partices, this pressure become equal to (p2 – p1) and if hypotonic solution is replaced by pure solvent then pressure becomes equal to p2.
Note : Osmotic pressure of very dilute solutions is also quite significant. So, its measurement in lab is very easy.
Plasmolysis : When the cell is placed in solution having osmotic pressure greater than that of the cell sap, water passes out of the cell due to osmosis. Consequently, cell material shrinks gradually. The gradual shrinking of the cell material is called plasmolysis.
Abnormal Colligative Properties : [Vant–Hoff correction :]
For electrolytic solutes the number of particles would be different from the number of particles actually added, due to dissociation or association of solute.
The actual extent of dissociation/association can be expressed with a correction factor known as vant Haff factor (i).
Vant–Hoff factor : i =![]()
If solute gets associated or dissociated in solution then experimental / observed / actual value of colligative property will be different from theoretically predicted value so it is also known as abnormal colligative property.
This abnormality can be calculated in terms of Vant-Hoff factor.
i=![]() =
=![]() =
= ![]()
i > 1 dissociation
i < 1 association
i = ![]()
Modified formula \ p= iCRT
p= (i1C1 + i2C2 + i3C3.....) RT
Case - I : Electrolyte dissociates
Relation between i & (degree of dissociation) :
Let the electrolyte be AxBy
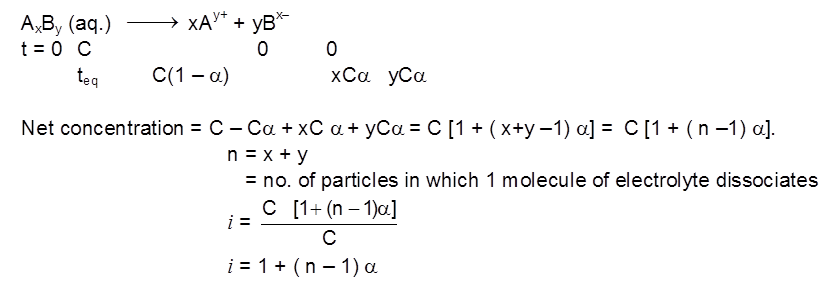
e.g. NaCl (100% ionised), i = 2. ; BaCl2 (100% ionised), i = 3. ; K4[Fe(CN)6] (75% ionised), i = 4.
Case - II : Electrolyte associates
Relation between degree of association b & i.
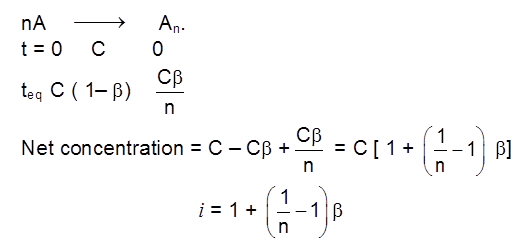
if dimerise n = 2 ; trimerise n = 3 ; tetramerise n = 4.
e.g. CH3COOH 100% dimerise in benzene, i = ![]() ; C6H5COOH 100% dimerise in benzene, i =
; C6H5COOH 100% dimerise in benzene, i = ![]()
(ii) Relative lowering in vapour pressure ( RLVP) :
Vapour Pressure :
The conversion of a liquid to a vapour takes place in a
visible way when the liquid boils, it takes place under all conditions.
say 5 gm liquid left at teq
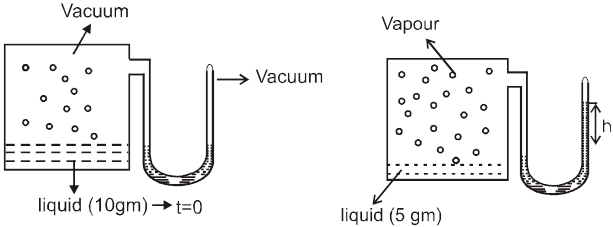
At eq. : the rate of evaporation = rate of condense
H2 O (λ) ![]() H2 O (g)
H2 O (g)
Kp = ![]() eq.
eq.
finally
The pressure exerted by vapours of the liquid when equilibrium is established between vapours & its liquid. is known as vapour pressure.
Vapour pressure is an equilibrium constant (KP) of the reaction.
liquid ![]() vapours.
vapours.
Since vapour pressure is an equilibrium constant so its value is dependent only on temperature for a particular liquid
It does not depends on the amount of liquid taken or surface area of the liquid or on volume or shape of the container. It is a characteristic constant for a given liquid.
Vapour Pressure of a solution
Vapour Pressure of a solutions of a non volatile solute ( solid solute ) is always found to be less than the vapour pressure of pure solvent .
Reason : Some of the solute molecules will occupy some surface area of the solutions so tendency of the solvent particles to go into the vapour phase is slightly decreased hence Pº > PS, where Pº is vapour pressure of pure solvent and PS is vapour pressure of the solution.
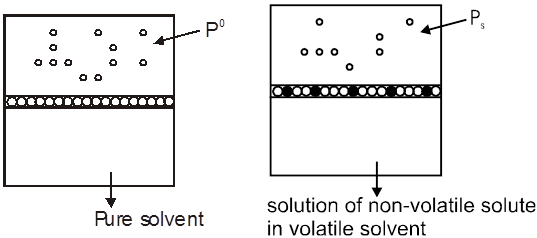
Lowering in VP = Pº – PS = DP
and Relative lowering in Vapour Pressure = ![]()
Raoult's law (For non–volatile solutes): The vapour pressure of a solution of a non-volatile solute is equal to the vapour pressure of the pure solvent at that temperature multiplied by its mole fraction.
OR Relative Lowering in Vapour Pressure = mole fraction of the non volatile solute in solution
.![]()
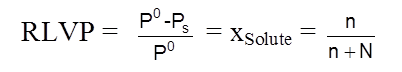
where n = number of moles of non-volatile solute and N = number of moles of solvent in the solution.
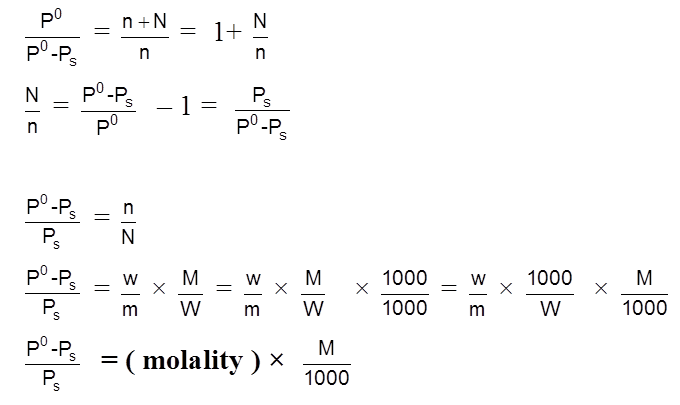
where w and W = mass of non-volatile solute and volatile solvent respectively
m and M = molar mass of non-volatile solute and volatile solvent respectively
If solute gets associated or dissociated ;
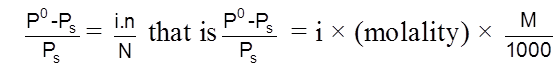
(iii) Elevation in Boiling point (DTb)
Boiling point of a Liquid : The temprature at which vapour pressure of a liquid becomes equal to the external pressure present at the surface of the liquid is called b.p of liquid at that pressure.
Normal boiling point : The temperature at which boiling ocuurs when the external pressure is exactly 1 atm is called the normal boiling point of the liquid. (Tb)
Boiling pt of any solution :
Since V.P. of solution is smaller then v.p. of pure solvent at any temperature hence to make it equal to Pext. we have to increase the temp. of solution by greater amount in comparision to pure solvent.
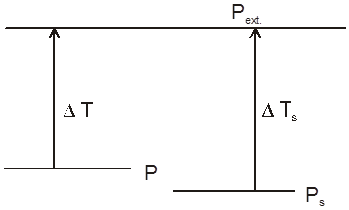
DHvapour of solvent =DHvapour solution
Graphical : 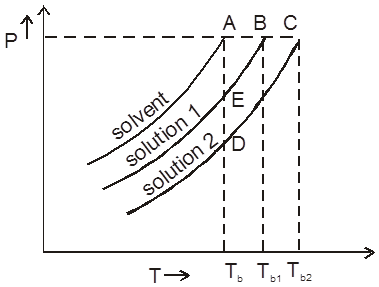
Using Raoult’s law :
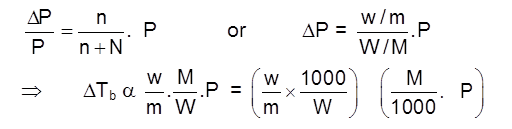
Tb = Kb × molality
Kb is dependent on property of solvent and known as molal elevation constant of solvent
It is also known as ebullioscopic constant.
Kb = elevation in boiling point of 1 molal solution.
Units : ![]() = K kg mol–1
= K kg mol–1
Using thermodynamics : Kb =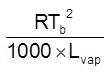
LVap – is latent heat of vapourization in cal/g or J/g
R = 2 cal mol–1 K–1 or 8.314 J mol–1 K–1. ; Tb = b.p. of liquid (in kelvin)
Kb = K kg mol–1
Also Kb = ![]()
Here:DHvap=molar enthalpy of vaporisation in cal/mole of J/mole
M Þ mole wt. of solvent
Lvap = 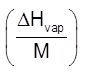
If solute gets associated/dissociated :DTb = i × Kb × molality
(iv) Depression in freezing pt (DTf)
Freezing point : Temperature at which vapour pressure of solid becomes equal to v.p of liquid is called freezing point of liquid or melting point of solid.
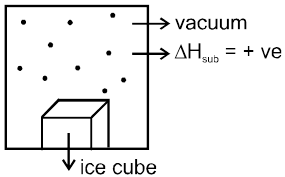
DHsub > DHvap.
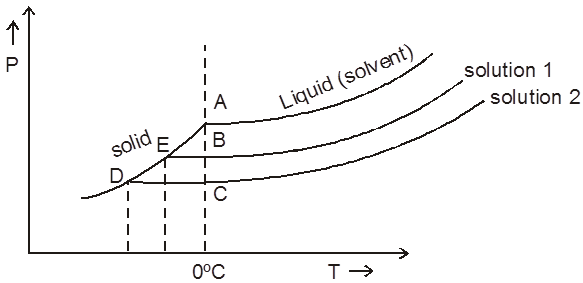
For - dil. solutions BE & CD can be assumed to be straight lines.
using similar triangles
\ DTf a P
\ DTf = Kf . Molality
Kf = molal depression constant = cryoscopic constant
Kf = 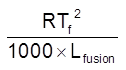 =
= 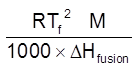
for water Tf = 273 K & LFusion = 80 cal / gm
Kf = ![]() = 1.86 K kg mol–1
= 1.86 K kg mol–1
At freezing point or below it only solvent molecules will freeze not solute molecules (solid will be of pure solvent)

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
