- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Classification of colloids
1. On the basis of physical state of D.P. and D.M.
On the bases of physical state of D.P. and D.M. colloidal solution may be divided into eight system.
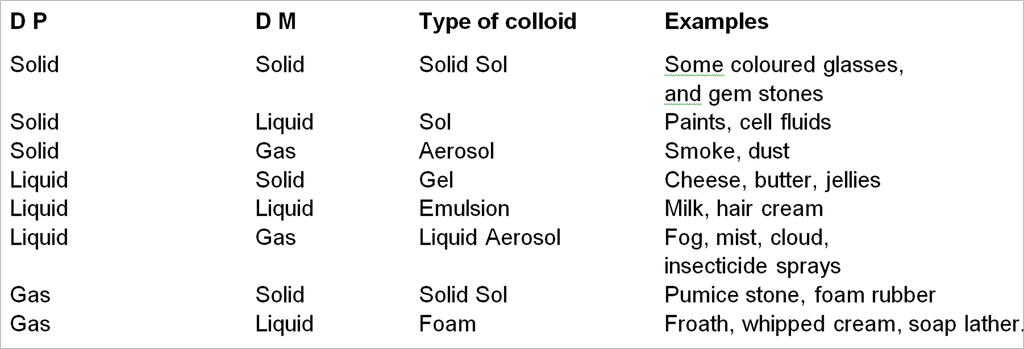
* Solution of gas in gas is not a colloidal system because it form homogeneous mixture.
2. On the Basis of interaction of D.P. for D.M. : There are two types-
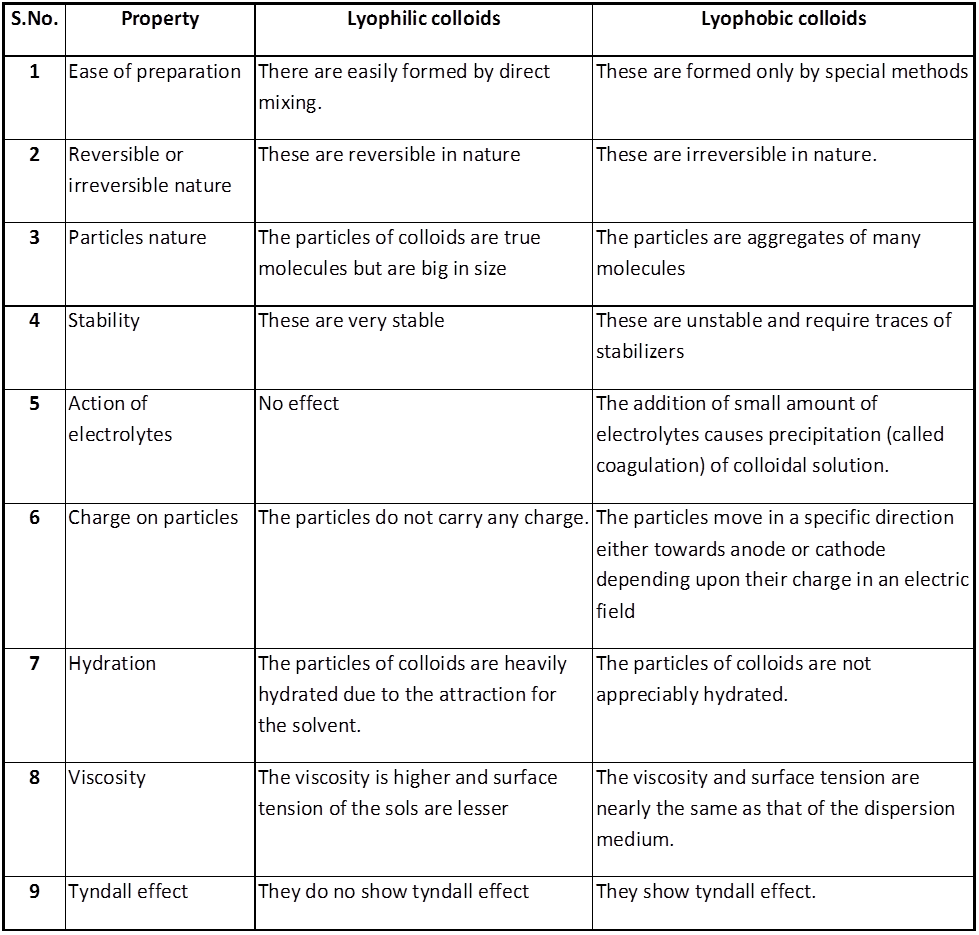
(i) Lyophilic colloids / liquid loving sols / intrinsic colloid. The colloidal solution in which the particles of the dispersed phase have a great affinity (or love) for the dispersion medium, are called lyophilic colloids. These solutions are easily formed and the lyophilic colloids are reversible in nature. In case water acts as the dispersion medium, the lyophilic colloid is called hydrophilic colloid. The common examples of lyophilic colloids are glue, gelatin, starch, proteins, egg albumin, rubber, etc.
(ii) Lyophobic colloids / solvent hating colloid / extrinsic colloid. The colloidal solutions in which there is no affinity between particles of the dispersed phase and the dispersion medium are called lyophobic colloids. Such solutions are formed with difficulty only by special methods. These sols are readily precipitated (or coagulated) on the addition of small amounts of electrolytes, by heating or by shaking and hence are not stable. Further, once precipitated, they do not give back the colloidal sol by simple addition of the dispersion medium. Hence these sols are also called irreversible sols. They need stabilising agents for their preservation. In case the dispersion medium is water, the lyophobic sol is called hydrophobic colloid. For example, the solution of metals like Ag and Au, hydroxides like Al (OH)3, Fe(OH)3, metal sulphides like As2S3 etc.
* Lyophilic sols are more stable than lyophobic sols, the additional stability is due presence of an envelope of the solvent layer (say water) around the colloidal particle, the process is known as hydration, To coagulate a hydrophilic sols we have to add a dehydrating agent in addition to electrolyte.
distinction between lyophilic and lyophobic colloids
3. On the basis of charge of on particles
(i) positive Sol
(a) Metal Oxide & Hydroxide - SnO2, TiO2, Fe2O3, AI(OH)3, Fe(OH)3, Cr(OH)3.
(b) Basic Dyes Methylene blue, vismark brown.
(ii) negative Sol -
(a) Metal sol - Ag, Au, Pt, Cu
(b) Acidic dye - congo red, eosin
(c) Sulphide Sol- CdS, HgS, As2S3, Sb2S3.
(d) Natural sol - Blood, clay, charcoal, latex rubber, dust particle in water, starch carbon particle in smoke, gum.
4. Other type of colloids : Multimolecular, macromolecular and associated colloids
Multimolecular colloids : In this type, the particles consist of an aggregate of atoms or small molecules size less than 1 nm. For example, sols of gold atoms and sulphur (S8) molecules. In these colloids, the particles are held together by van der Waal’s forces.
Macromolecular colloids : In this types, the particles of the dispersed phase are sufficiently big in size (macro) to be of colloidal dimensions. These macromolecules forming the dispersed phase are generally polymers having very high molecular masses. These colloids are quite stable and resemble true solutions in many respects. Naturally occuring macromolecules are starch, cellulose, proteins, enzymes, gelatin, etc.
Associated colloids (Micelles): These are the substances which behave as normal strong electrolytes at low concentration but behave as colloidal particles at higher concentration. These associated particles are also called micelles. Ex. Soap.
Micelles : There are some substances which at low concentrations behave as normal strong electrolytes but at higher concentrations exhibit colloidal behaviour due to the formation of aggregated particles. The aggregated particles thus formed are called micelles. These are also known as associated colloids. The formation of micelles takes place only above a particular temperature called Kraft Temperature (Tk) and above a particular concentration called Critical Micelle Concentration (CMC). On dilution, these colloids revert back to individual ions. Surface active agents such as soaps and synthetic detergents belong to this class. For soaps, the CMC is ~ 10–4 to 10–3 mol L–1. These colloids have both lyophobic and lyophilic parts. Micelles may contain as many as 100 molecules or more.
Mechanism of micelle formation: Let us take the example of soap solutions. Soap is sodium salt of a higher fatty acid and may be represented as RCOO–Na+ e.g., sodium stearate viz.CH3(CH2)16COO– Na+ which is a major component of many bar soaps. When dissolved in water, it dissociates into RCOO- and Na+ ions. The RCOO– ions, however, consist of two parts i.e., long hydrocarbon chain R (also called non-polar 'tail') which is hydrophobic (water repelling) and the polar group COO– (also called polar-ionic 'head') which is hydrophilic (water loving). The RCOO– lons are, therefore, present on the surface with their COO– groups in water and the hydrocarbon chains R staying away from it, and remain at the surface, but at higher concentration these are pulled into the bulk of the solution and aggregate in a spherical form with their hydrocarbon chains pointing towards the centre with COO– part remaining outward on the surface. An aggregate thus formed is known as ‘Ionic micelle'. These micelles may contain as many as upto 100 such ions.
Aggregation of RCOO– ions to form an ionic micelle.
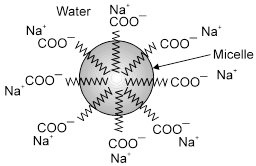
Similarly, in case of detergents, e.g., sodium lauryl sulphate viz. CH3(CH2)11SO4– Na+, the polar group is – SO4– along with the long hydrocarbon chain. Hence, the mechanism of micelle formation is same as that of soaps.
Critical micelle concentration [CMC] : The minimum concentration required for micelle formation is called critical micelle concentration. Its value depends upon the nature of D.P. and D.M. For eg. Surface active agent (surfactants, which decrease the surface tension) like soaps and detergents form micelle beyond CMC (~10–3 mol/litre for soaps).
* Usually longer the hydrophobic chain, smaller is its CMC.
* Also CMC increase with decreasing polarity of the D.M.
* The micelles ‘formation takes place only above a particular temperature called as Kraft Temperature (Tk ).
*At CMC, the micelles are spherical in shape, but that start flattening with increase in concentration and ultimately form sheet or film like structures which have a thickness of two molecules. These are called lamelar micelles or McBain Micelles.
Example of micelles :
(i) Sodium stearate C17H35COO–Na+(Soap).
(ii) Sodium lauryl sulphate CH3 [CH2]11 SO4– Na+ (Detergent).
(iii) Cetyl trimethyl ammonium bromide (Detergent). CH3(CH2)15N (CH3)3Br–.
(iv) Sodium p-dodecylbenzenesulphonate (Detergent) : ![]()
(v) Acidic (negative colloids) and basic (positive colloids) dyes.The Cleansing Action of Soaps : It has been mentioned earlier that a micelle consists of a hydrophobic hydrocarbon like central core. The cleansing action of soap is due to these micelles, because oil and grease can be solubilised in their hydrocarbon, like centres which are not otherwise soluble in water. This is shown diagrammatically in Figure The dirt goes out along with the soap micelles.
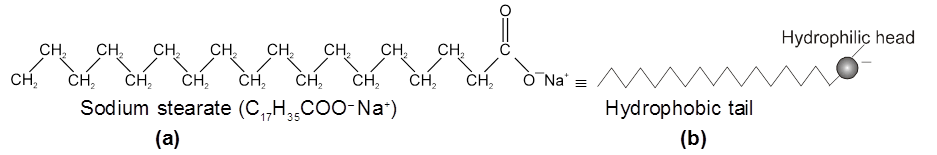
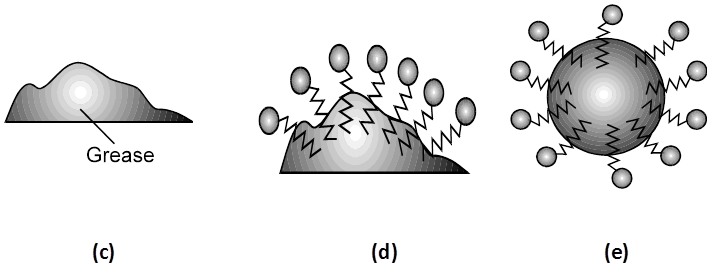
(a) A sodium stearate molecule
(b) The simplified representation of the molecule that shows a hydrophilic head and a hydrophobic tail
(c) Grease (oily substance) is not soluble in water
(d) When soap is added to water, the non-polar tails of soap molecules dissolve in grease
(e) Finally, the grease is removed in the form of micelles containing grease.
*Surfactants : They can be ionic as well as non-ionic. The ionic are soaps and detergent. The surfactant gets adsorbed at the interface between the dispersed droplets and dispersion medium in a form of mono molecular layer and lowers the interfacial tension between oil and water so as to facilitate the mixing of two liquids.
Preparation of lyophobic colloidal sols :
[A] Condensation methods :
In these methods particles of atomic or molecular size are induced to combine to form aggregates having colloidal dimensions. For this purpose chemical as well as physical methods can be applied.
(a) Chemical methods. Colloidal solutions can be prepared by chemical reactions leading to formation of molecules by double decomposition, oxidation, reduction or hydrolysis. These molecules then aggregate leading to formation of sols.
(i) Double decomposition : When a hot aqueous dilute solution of arsenous oxide (As2O3) is mixed with a saturated solution of H2S in water, a colloidal sol of arsenous sulphide (As2S3) is obtained.
As2O3(in hot water) + 3H2S (saturated solution in H2O) ![]() As2S3(sol) + 3H2O
As2S3(sol) + 3H2O
(ii) Oxidation : A colloidal sol of sulphur is obtained by passing H2S into a solution of sulphur dioxide.
SO2 + 2H2S(saturated solution in H2O) ![]() 3S(sol) + 2H2O
3S(sol) + 2H2O
Sulphur sol can also be obtained when H2S is bubbled through Br2 water or nitric acid (oxidizing agent).
(iii) Reduction : Colloidal sol of metals like gold, silver solution are obtained by following method.
2 AuCl3 + 3 HCHO + 3H2O 2Au(sol) + 3HCOOH + 6HCl.
(purple of cassius)
(iv) Hydrolysis : A colloidal sol of metal hydroxides like Al(OH)3 or Cr(OH)3 is obtained by boiling a dilute solution of FeCl3 , AlCl3 or CrCl3 .
FeCl3 + 3H2O Fe(OH)3 (sol) + 3HCl
AlCl3 + 3H2O Al(OH)3 (sol) + 3HCl
The colloidal sol of sillicic acid is also obtained by hydrolysis of dilute solution of sodium silicate with hydrochloric acid.
Na4SiO4 + 4HCl ![]() Si(OH)4 (sol) + 4NaCl.
Si(OH)4 (sol) + 4NaCl.
(b) Physical methods : The following physical methods are used to prepare the colloidal solutions.
(i) By Exchange of solvent : When a true solution is mixed with an excess of the other solvent in which the solute is insoluble but solvent is miscible, a colloidal sol is obtained. For example,
when a solution of sulphur in alcohol is poured in excess of water, a colloidal sol of sulphur is obtained.
when a solution of phenolphthalein in alcohol is poured in excess of water a white sol of phenolphthalein is found.
Phenolphthalein, I2 , sulphur sol can be prepared by this methods.
(ii) Excessive cooling : Molecules of certain substance condense together on excess cooling to form colloidal size particle. The colloidal sol of ice in an organic solvent such as CHCl3 or ether can be obtained by freezing a solution of water in the solvent. The molecules of water which can no longer be held in solution separately combine to form particles of colloidal size.
[B] Dispersion Methods : In these methods large particles of the substance are broken into particles of colloidal dimensions in the presence of dispersion medium. These are stabilized by adding some suitable stabilizer. Some of the methods employed are given below :
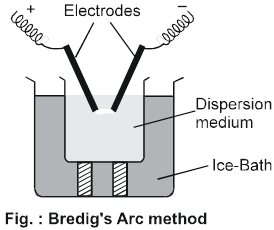
(a) Electrical disintegration or Bredig's Arc method : This process involves dispersion as well as condensation. Colloidal sols of less reactive metals such as gold, silver, platinum, copper, lead etc., can be prepared by this method. In this method, electric arc is struck between electrodes of the metal immersed in the dispersion medium as shown in fig. The intense heat produced vaporises the metal, which then condenses to form particles of colloidal size by surrounding cooling mixture (ice).
*A slight trace of KOH is added in water to stabilized colloidal solutions.
(b) Peptization: The term has originated from the digestion of proteins by the enzyme pepsin. Peptization may be defined as (the process of converting a precipitate into colloidal sol by shaking it with dispersion medium in the presence of a small amount of electrolyte)

The electrolyte used for this purpose is called peptizing agent. This method is applied, generally, to convert a freshly prepared precipitate into a colloidal sol. During peptization, the precipitate adsorbs one of the ions of the electrolyte on its surface. The ion adsorbed on the surface is common either with the anion or cation of the electrolyte. This causes the development of positive or negative charge on precipitates which ultimately break
up into smaller particles having the dimensions of colloids.
For example :
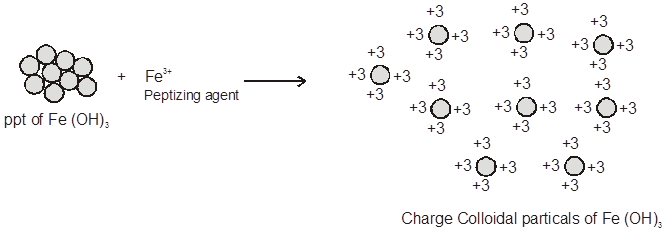
(i) When freshly precipitated Fe(OH)3 is shaken with aqueous solution of FeCl3 (peptizing agent) it adsorbs Fe3+ ions and thereby breaks up into small-sized particles.
FeCl3 ![]() Fe3+ + 3Cl– ;
Fe3+ + 3Cl– ;
Fe(OH)3 + Fe3+ ![]() Fe(OH)3 | Fe3+
Fe(OH)3 | Fe3+
(ii) Freshly prepared stannic oxide on treatment with a small amount of dilute hydrochloric acid forms a stable colloidal sol of stannic oxide, SnO2 ; Sn4+ .
SnO2 + 4HCl ® Sn4+ + 2H2O + 4Cl–
SnO2 + Sn4+ ® SnO2 / Sn4+ .
(iii) Freshly precipitated silver chloride can be converted into a colloidal sol by adding a small amount of hydrochloric acid, AgCl : Cl– .
(iv) Cadmium sulphide can be peptised with the help of hydrogen sulphide, CdS : S2– .
Purification of Colloidal Sols : The colloidal sols obtained by various methods are impure and contain impurities of electrolytes and other soluble substances. These impurities may destabilise the sol. Hence, they have to be removed. A very important method of removal of soluble impurities from sols by a semipermeable membrane is known as dialysis.
A. Dialysis : It is a process of removing a dissolved substance from a colloidal solution by means diffusion through suitable membrane. Since particles in true solution (ions or smaller molecules) can pass through animal membrane or parchment paper or cellophane sheet but colloidal particle do not, the appratus used for this purpose is called Dialyser.
A bag of suitable membrane containing the colloidal solutions is suspended in a vessel through which fresh water continously flow. The molecules and ions (crystalloids) diffuse through membrane into the outer water & pure colloidal solution is left behind.
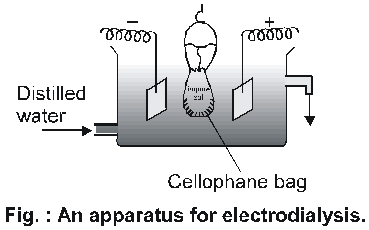
Movement of ions across the membrane can be expedited by applying electric potential through two electrodes as shown in fig.
This method is faster than simple dialysis and is known as
Electrodialysis.
*The most important applications of dialysis is in the purification of blood in the artificial kidney machine. In case of kidney failure, blood cannot be purified. under such condition, the blood is separated from dissolved toxic impurities by dialysis and re-introduced in the bloods stream.
*Dialysis is not applicable for non-electrolytes like glucose, sugar, etc.
B. Ultra Filtration : In this method, colloidal sols are purified by carrying out filtration through special type of graded filters called ultra-filters. These filter papers allow only the electrolytes to pass through. These filter papers are made of particular pore size by impregnating with colloidal solution and subsequently hardened by soaking in formaldehyde collodion. In order to accelerate the filtration through such filter papers, increased pressure or section is employed.
Important properties of colloidal sols :
Heterogeneous character :
Colloidal sols are heterogeneous in character as they consist of two phases.
(a) dispersed phase and (b) dispersion medium.
Visibility : Due to scattering caused by the colloidal particles, it will appear as a bright spot moving randomly.
Filterability :Colloidal particles pass through an ordinary filter paper. However, the particle do not pass through other fine membranes.
Colligative Properties : Colloidal sols show the colligative properties viz. relative lowering of vapour pressure, elevation in boiling point, depression in freezing point and osmotic pressure. However, due to high average molecular masses of colloidal particles, mole fraction of the dispersed phase is very low. Hence, the values of the colligative properties observed experimentally are very small. Only osmotic pressure measurements are used in determining the molecular mass of polymers.
Optical Properties-Tyndall effect : Tyndall, in 1869, observed that if a strong beam of light is passed through a colloidal sol placed in a dark place, the path of the beam gets illuminated. This phenomenon is called Tyndall effect, which is due to the scattering of light by the colloidal particles. The illuminated path of beam is called Tyndall cone. This phenomenon is due to scattering of light from the surface of colloidal particles. In a true solution there are no particles of sufficiently large diameter to scatter light & hence the beam is invisible.
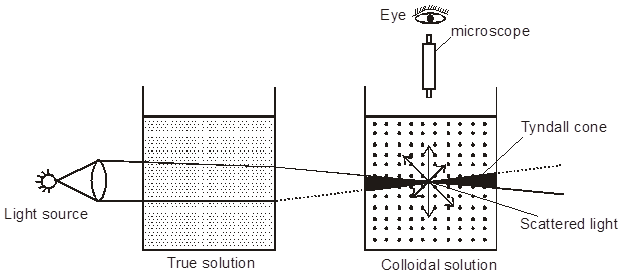
*The intensity of scattered light depends on the difference between the refractive indice of the D.P and D.M., In lyophobic colloids, this difference is appreciable and therefore the tyndal effect is quite well defined but in lyophilic sols the difference is very small and the tyndal effect is very weak. Thus in sols of silicic acid, blood serum, albumin, etc. there is little or no tyndal effect.
Example of Tyndall Effect
® Blue colour of sky and sea water.
® Visibility of tail of comets.
® Light thrown from a projector in cinema hall.
® Appearance of dust particle in a semi darked room.
Application of Tyndall Effect :
(i) In making ultramicroscopes.
(ii) In finding heterogenity of solution.
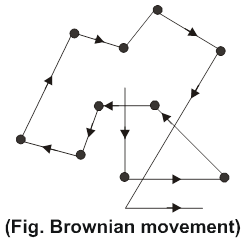
Brownian movement: Robert Brown, a botanist, discovered in 1827 that pollen grains placed in water do not remain at rest but move about continuously and randomly. Later on, this phenomenon was observed in case of colloidal particles when they were seen under an ultramicroscope. The particles were seen to be in constant zig-zag motion as shown in fig. This zig-zag motion is called Brownian movement.
Brownian movement arises because of the impact of the molecules of the dispersion medium with the colloidal particles. It has been postulated that the impact of the molecules of the dispersion medium on the colloidal particle are unequal leading to zig-zag motion. However, as the size of the particle increases, the effect of the impacts average out and the Brownian movement becomes slow. Ultimately, when the dispersed particle becomes big enough to acquire the dimensions of suspension, no Brownian movement is observed.
Factors Affecting Brownian Movement :
(i) If particles is large then brownian movement becomes less.
(ii) Brownian movement increases with increasing temperature.
(iii) The brownian movement does not change with time & remains same for months or even for a year.
Important :
(i) In confirmation of kinetic energy.
(ii) Determination of Avogadro numbers.
(iii) Stability of colloidal solution : Brownian movement does not allow the colloidal particles to settle down to gravity & thus is responsible for their stability.
Electrical Properties (Electrophoresis) : The particles of the colloids are electrically charged and carry positive or negative charge. The dispersion medium has an equal and opposite charge making the system neutral as a whole. Due to similar nature of the charge carried by the particles, they repel each other and do not combine to form bigger particles. That is why, a sol is stable and particles do not settle down. Arsenious sulphide, gold, silver and platinum particles in their respective colloidal sols are negatively charged while particles of ferric hydroxide, aluminium hydroxide are positively charged. The existence of the electric charge is shown by the phenomenon of electrophoresis. It involves the 'movement of colloidal particles either towards the cathode or anode, under the influence of the electric field'. The apparatus used for electrophoresis as shown in fig.

The colloidal solution is placed in a U-tube fitted with platinum electrodes. On passing an electric current, the charged colloidal particles move towards the oppositely charged electrode. Thus, if arsenic sulphide sol is taken in the U-tube, in which negatively charge particle of arsenic sulphide move towards the anode.
*Earlier this process was called cataphoresis because most of the colloidal sols studied at that time were positively charged and moved towards cathode.
Isoelectric point : The H+ concentration at which the colloidal particles have no charge is known as the isoelectric point. At this point stability of colloidal particles becomes very less & do not move under influence of electric field.
Fe(OH)3 sol prepared by the hydrolysis of FeCl3 solution adsorbs Fe3+ and this is positively charged.
FeCl3 + 3H2O ![]() Fe(OH3) + 3HCl
Fe(OH3) + 3HCl
Fe(OH)3 + FeCl3 ® Fe(OH)3 ![]() Fe3+ : 3Cl–
Fe3+ : 3Cl–
Fixed part Diffused part.
Positive charge on colloidal sol is due to adsorption of Fe3+ ion (common ion between Fe(OH)3 and FeCl3).
As2S3 colloidal sol is obtained when As2O3 is saturated with H2S :
As2O3 + 3H2S ®As2S3 + 3H2O.
As2S3 adsorbs S2– ions (common between H2S and As2S3 and thus is negatively charged).
As2S3 + H2S ® As2S3 ![]() S2– : 2H+.
S2– : 2H+.
AgI in contact with AgNO3 forms positively charged colloidal sol due to adsorption of Ag+ ion.
AgI + AgNO3 ® [AgI]Ag+ : NO3– , AgI in contact with KI forms negatively charged colloidal sol due to adsorption of I– ion AgI + KI ® AgI  I– : K+.
I– : K+.
SnO2 in acidic medium forms positively charged colloidal sol due to adsorption of Sn4+ formed.
SnO2 + 4H+ ® Sn4+ + 2H2O
SnO2 + Sn4+ ® SnO2 ![]() Sn4+
Sn4+
SnO2 in alkaline medium forms negatively charged colloidal sol due to adsorption of SnO32– formed.
SnO2 + 2OH– ® SnO32– + H2O
SnO2 + SnO32– ® SnO2 ![]() SnO32–
SnO32–
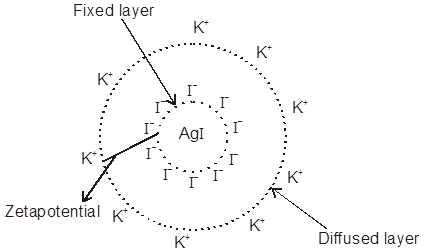
Electric Double Layer Theory or Helm-holtz Electric double layer :
The surface of colloid particles acquire a positive or negative charge by the selective (preferential) adsorptions of common ions carrying positive or negative charge respectively to form first layer. This layer attract counter ions from D.M. form a second layer. The combination of two layers of opposite charge around the colloidal particle is called Helm-holtz electric double layer. The first layer of ions is firmely held and is termed as fixed layer while the second layer is mobile which is termed as diffused layer. The charge of opposite ions of fixed and diffused layer double layer results in a difference in potential between two opposite charge layer is called the electrokinetic potential or zetapotential which can be given by
Example :
When silver nitrate solution is added to KI solution, the precipitation of AgI adsorb iodide ions from the D.M with the formation of fixed layer and negatively charged colloidal solution form, however when KI solution is added to AgNO3 solution positive charge sol result due to the adsorbs of Ag+ ions from D.M.
AgI/I– AgI/Ag+
Negative charged Positively charged.
This fixed layer attracts counter ions from the medium forming a second layer.
Coagulation/Flocculation : The presence of small amounts of appropriate electrolytes is necessary for the stability of the colloids. However, when an electrolyte is added in larger concentration; the particles of the sol take up the ions which are oppositely charged and thus get neutralised. The neutral particles then start aggregating giving particles of larger size which are then precipitated. This process of aggregation of colloidal particles into an insoluble precipitate by the addition of some suitable electrolyte is known as coagulation. At lower concentration of electrolytes, the aggregation of particles is called flocculation that can be reversed on shaking while at higher concentration of electrolyte, coagulation takes place and the same cannot be reversed simply by shaking. The stability of the lyophobic colloids is due to presence of charge on colloidal particles. If, somehow, the charge is removed, the particles will come near to each other to form aggregates and settled down under the force of gravity.
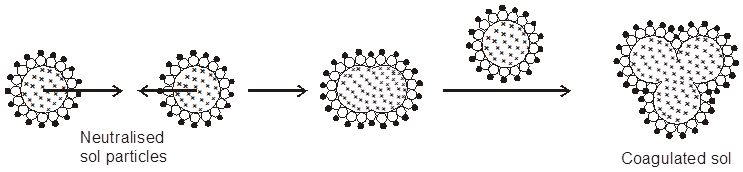
Coagulation of lyophobic sols can be carried out by the following methods.
(i) By electrophoresis
(ii) By mutual precipitation : It is a process in which oppositely charged sol are mixed in proper proportion to neutralise the charge of each other causing coagulation of both the sol.
Example : Positively charged Fe(OH)3 and negatively charged As2S3 colloidal particle containing sol on mixing get coagulated.
(iii) By Prolonged Dialysis : On prolonged dialysis, traces of the electrolyte present in the sol are removed almost completely and the colloidal unstable and ultimately coagulate.
(iv) By Boiling : Sols such as sulphur and silver halides dispersed in water may be coagulated by boiling because increased collisions between sol particle and the water molecule removed the adsorbed electrolytes. This takes away the charge from the particles and helps them to coagulate.
(v) By cooling : Certain sol can also be coagulated by lowering temperature. For example, accumulation of cream on the surface of milk on cooling. This is because at lower temperature the dispersion medium molecules do not exert sufficient force on to the dispersed particles and hence the Brownian motion becomes less effective.
(vi) By the addition of electrolyte : When excess of an electrolyte is added, the colloidal particles are precipitated.
Coagulation value or Flocculation value : It needs to be noted that the coagulation of a colloidal solution by an electrolyte does not take place until the added electrolyte has certain minimum concentration in the solution. The minimum concentration of electrolyte in millimoles required to cause coagulation of one litre of colloidal solution is called coagulation value. It is express in terms of millimoles/litre.
Coagulation value = 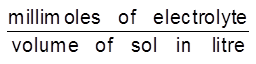
Comparision of relative coagulating power of two electrolyte for the same colloidal solution :
The coagulation value decrease with increase in charge of the coagulating ion.
Coagulating power µ ![]() .
.
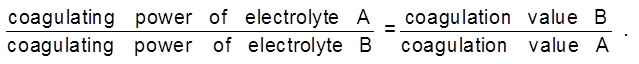
Hardy-Schulze Rule : According to this rule greater is the valency of coagulating ion, greater its power to cause precipitation. This is known as Hardy-Schulze.
In case of positive charged sol, the coagulating power of anion is in the order of [Fe(CN)6]4– > PO43– > SO42– > Cl–
In case of negative charged sol, the coagulating power of cation is in the order of Al3+ > Ba2+ > Na+.
The coagulating power of bivalent ion is 20-80 times higher than monovalent ion and coagulating power of trivalents is many times more than bivalent.
Protective colloidal sols : Lyophilic colloidal sols are much more stable than lyophobic colloidal sols. This is due to the extensive solvation of lyophilic colloidal sols, which forms a protective layer outside it and thus prevents it from forming associated colloids. Lyophobic sols can easily precipitate by addition of small amount of an electrolyte. They can be prevented from coagulation by previous addition of some lyophilic colloid. This is due to formation of a protective layer by lyophilic sols outside lyophobic sols. Process of protecting the lyophobic colloid solution from precipitation by an electrolyte due to previous addition of some lyophilic colloid is called protection of colloid and lyophilic colloidal sols are called protective sols.
Eg : Gelatin, Sodium caseinate, Egg albumin, Gum arabic, Potato starch etc.,
Gelatin (lyophilic) protects gold sol (lyophobic) colloids is expressed in terms of gold number.
Gold Number : Zpsigmondy (1901) introduce a term called gold number it is defined as ‘’the minimum amount of the protective colloid in milligrams which when added to 10 ml of a standard gold sol is just sufficient to prevent a colour change from red to blue on the addition of 1 ml of 10% sodium chloride solution. It may be noted that smaller of the gold number, greater will be protecting power of the protective colloid.
Protecting power µ . 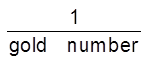
Gold Number = 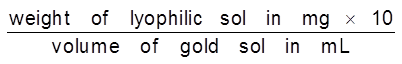
The gold numbers of a few protective colloids are as follows :
![]()
Uses of protective action :
(i) Gelatin is added in the preparation of ice cream to protect the particle of ice.
(ii) Protargol and Argyrol, is a silver sol protected by organic material used as eye drop.
Applications of Colloids : Colloids including emulsions find a number of uses in our daily life and industry. Some of the uses are given below.
n medicines : A wide variety of medicinal and pharmaceutical preparations are emulsions. Colloidial medicines are easily adsorbed by the body tissue because of large surface area.
* Colloidal antimony is used in curing kalaazar.
* Milk of magnesia, an emulsion, is used for stomach disorder.
* Colloidal gold is used for intramuscular injection.
* Colloidal sulphur are used as Germicides.
* Argyrol is a silver sol used as an eye lotion.
* Colloidal Fe(OH)3 is given to arsenic poisoning patients as it adsorbs arsenic and then gets omited out.
Tanning : Animal hides are colloidal in nature. Which contain positive charge colloidal particles of protein. This hide is kept in a tank containing tannic acid, which contains negatively charge colloidal particle. Therefore, mutual coagulation takes place this results in hardening of leather, this process is termed as tanning of leather. Chromium salts are also used in place of tannic acid.
Photographic plate & Film : Photographic plate or films are prepared by coating an emulsion of the light sensitive silver bromide in gelatin over glass plates or celluloid films. Gelatin prevent the coagulation of colloidal particle of AgBr.
Rubber plating : The negatively charged rubber particles from rubber sol are deposited on wares and handles of different tools. Rubber gloves are formed by rubber plating on suitable templates.
Sewage disposal : Sewage water contains charged colloidal particles of dirt, rubbish, etc., and these do not settle down easily. The particles can be removed by discharging them at electrodes. Dirty water is passed through a tunnel fitted with metallic electrodes which are maintained at high potential difference. The particles migrate to the oppositely charged electrode, lose their charge and get coagulated. The deposited matter is used as a manure and the water left behind is used for irrigation.
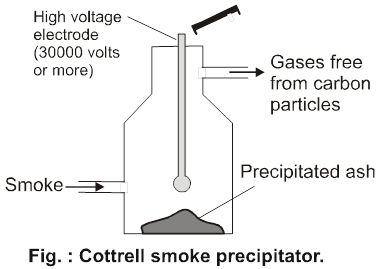
Cottrell smoke precipitator :Smoke is a dispersion of negatively charged colloidal particles of carbon in air and can be made free of these colloidal particles by passing it through cottrell precipitator as shown in fig. installed in the chimney of an industrial plant. It consists of two metal discs charged to a high potential. The carbon particles get discharged and precipitate, while gases come out from the chimney.
Formation of deltas : The river water contains colloidal particles of sand and clay which carry negative charge. The sea water contains +ve ions such as Na+, Mg2+, Ca2+, etc. As the river water meets sea water, these ions discharge the sand or clay particle which are precipitated in the form of delta.
Artificial rain : Cloud consists of charge particle of water disperse in air. Rain is caused by aggregation of these minute particles, artificial rain can be done by throwing electrified sand or Agl from aeroplanes, colloidal H2O particle present in cloud will get coagulated by these sand or Agl particles to form bigger water drops causing rain.
Stop bleeding from a cut : Blood is a colloidal solution containing a –ve charge colloidal particle (Albuminoid), bleeding can be stopped by use of alum or FeCl3 solution. The addition of Al3+ or Fe3+ causes coagulation of blood, so bleeding stops.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
