- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Ideal and Non-ideal Solution
Ideal Solutions : Those solutions which obey Raoult's law over the entire range of conc. are called ideal solutions. When the forces of attraction between A—A, B—B is similar to A—B, then A and B will form ideal solution.
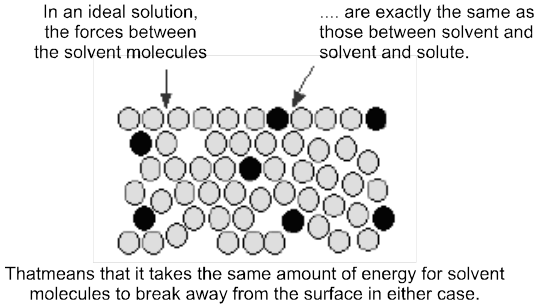
Properties of ideal solution :
(i) Raoult's law is obeyed
(ii) DHmix = 0, i.e., there should not be enthalpy change when components of ideal solutions are mixed.
(iii) DVmix = 0, (1L + 1L = 2L) i.e., there should not be change in volume on mixing. e.g.; n-hexane and n-heptane; ethyl bromide and ethyl iodide; benzene and toluene; chlorobenzene and bromobenzene form ideal solutions.
(iii) DSmix = +ve
(iv) DGmix = –ve
Non_Ideal Solutions :
Those solutions which do not obey Raoult's over the entire range of concentration are called non-ideal solutions.
When the forces of attraction between A—A, B—B is different from A—B then 'A' and 'B' form non-ideal solutions. For these solutions :
(i) Raoult's law is not obeyed.
(ii) DHmix ¹ 0 ;
(iii) DVmix ¹ 0.
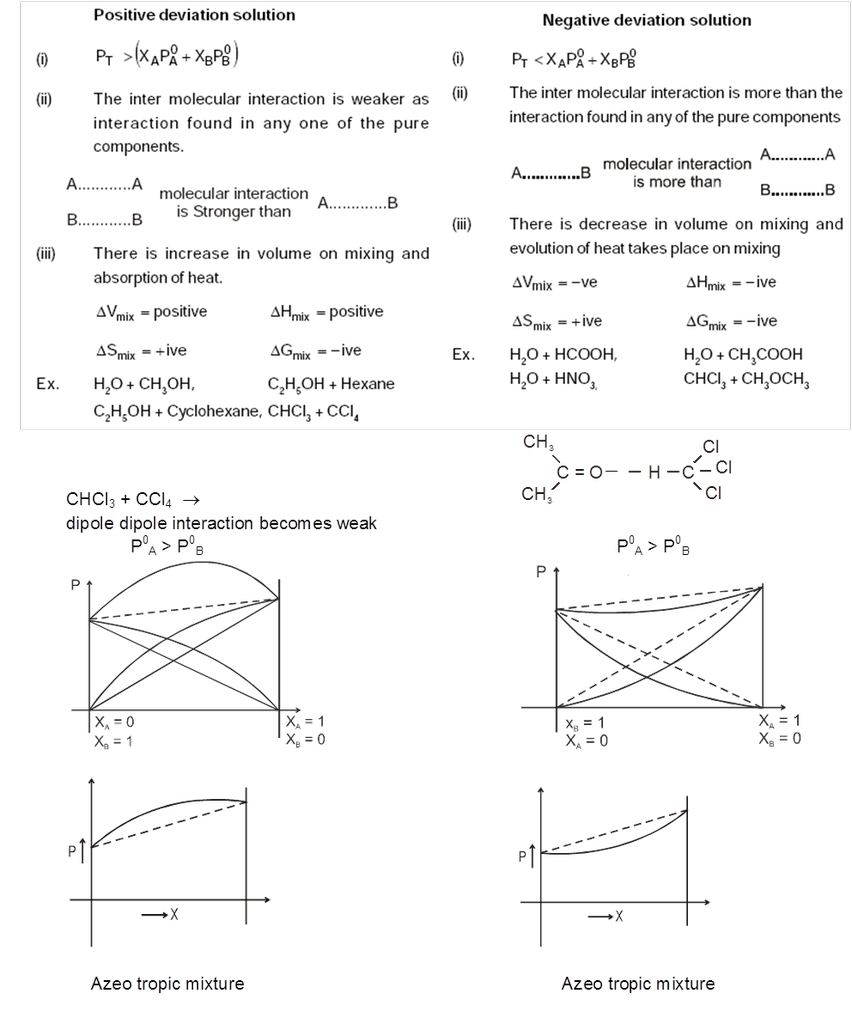
Types of Non-Ideal Solutions : Non-ideal solution can be two types.
- Non ideal solutions showing positive deviation
- Non ideal solutions showing negative deviation

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
