- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Group 18 Elements : The Zero Group Family
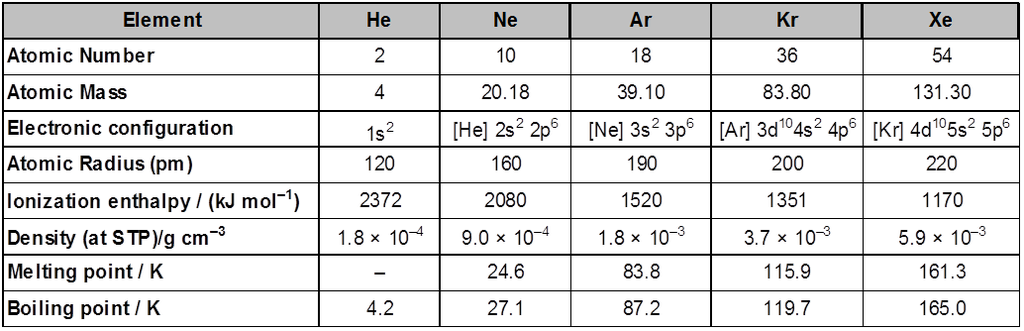
Group 18 consists of six elements: helium, neon, argon, krypton , xenon and radon . All these are gases and chemically unreactive. They form very few compounds . Because of this they are termed noble gases.
Occurrence : All the noble gases except radon occur in the atmosphere. Their atmospheric abundance in dry air is ~ 1% by volume of which argon is the major constituent. Helium and sometimes neon are found in minerals of radioactive origin e.g., pitchblende, monazite, cleveite. The main commercial source of helium is natural gas. Xenon and radon are the rarest elements of the group. Radon is obtained as a decay product of 226Ra.
![]()
Most abundant element in air is Ar. Order of abundance in the air is Ar > Ne > Kr > He > Xe.
Electronic Configuration : All noble gases have general electronic configuration ns2np6 except helium which has 1s2 . Many of the properties of noble gases including their inactive nature are ascribed to their closed shell structures.
Ionisation Enthalpy : Due to stable electronic configuration these gases exhibit very high ionisation enthalpy . However, it decreases down the group with increases in atomic size.
Atomic Radii : Atomic radii increase down the group with increase in atomic number.
Electron Gain Enthalpy : Since noble gases have stable electronic configurations, they have no tendency to accept the electron and therefore, have larger positive values of electron gain enthalpy.
Physical properties : All the noble gases are mono-atomic. They are colourless, and tasteless. They are sparingly soluble in water. They have very low melting and boiling points because the only type of interatomic interaction in these elements is weak dispersion forces,. Helium has the lowest boiling point (4.2K) of any known substance. It has a unusual property of diffusing through most commonly used laboratory materials such as rubber, glass or plastics.
Atomic & physical properties
Chemical Properties :
In general, noble gases are least reactive. Their inertness to chemical reactivity is attributed to the following reasons :
(i) The noble gases except helium (1s2) have completely filled ns2 np6 electronic configuration in their valence shell.
(ii) They have high ionisation enthalpy and more positive electron gain enthalpy.
The reactivity of noble gases has been investigated occasionally ever since their discovery, but all attempt to force them to react to form the compounds were unsuccessful for quite a few years. In March 1962, Neil Bartlett, then at the University of British Columbia, observed the reaction of a noble gas. First , he prepared a red compound which is formulated as O2+ PtF6–. He , then realised that the first ionisation enthalpy of molecular oxygen (1175 kJ mol –1) was almost identical with that xenon (1170 kJ mol–1). He made efforts to prepare same type of compound with Xe+ PtF6– by mixing Pt F6 and Xenon. After this discovery, a number of xenon compounds mainly with most electronegative elements like fluorine and oxygen, have been synthesised.
The compounds of krypton are fewer. Only the difluoride (KrF2) has been studied in detail. Compounds of radon have not been isolated but only identified (e.g., RnF2) by radiotracer technique. No true compounds of Ar, Ne or He are yet known .

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
