- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
The Lanthanides
The names, symbols, electronic configurations of atomic and some ionic states and atomic and ionic radii of lanthanum and lanthanide (for which the general symbol Ln is used) are given in Table.
Electronic Configurations : It may be noted that atoms of these elements have electronic configuration with 6s2 common but with variable occupancy of 4f level (Table). However, the electronic configurations of all the tripositive ions (the most stable oxidation state of all the lanthanides) are of the form 4fn (n = 1 to 14 with increasing atomic number).
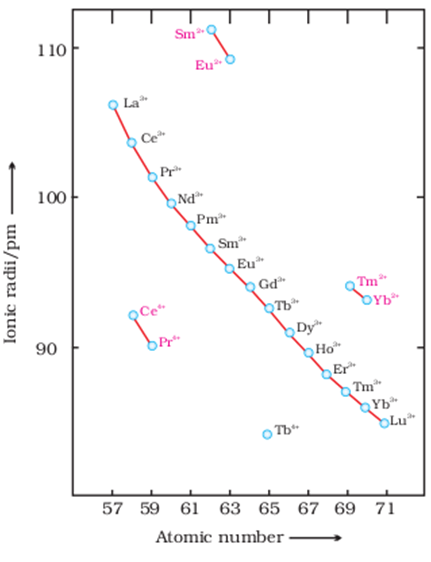
Atomic and Ionic Sizes : The overall decrease in atomic and ionic radii from lanthanum to lutetium (the lanthanide contraction). The shielding of one 4 f electron by another is less than one d electron by another with the increase in nuclear charge along the series. There is fairly regular decrease in the sizes with increasing atomic number. The cumulative effect of the contraction of the lanthanide series, known as lanthanide contraction, causes the radii of the members of the third transition series to be very similar to those of the corresponding members of the second series. The almost identical radii of Zr (160 pm) and Hf (159 pm), a consequence of the lanthanide contraction.
Oxidation States : In the lanthanides, La(III) and Ln(III) compounds are predominant species. However, occasionally +2 and +4 ions in solution or in solid compounds are also obtained.
This irregularity (as in ionisation enthalpies) arises mainly from the extra stability of empty, half-filled or filled f subshell. Thus, the formation of CeIV is favoured by its noble gas configuration, but it is a strong oxidant reverting to the common +3 state. The EΘ value for Ce4+/ Ce3+ is + 1.74 V which suggests that it can oxidise water. However, the reaction rate is very slow and hence Ce(IV) is a good analytical reagent.
Pr, Nd, Tb and Dy also exhibit +4 state but only in oxides, MO2. Eu2+ is formed by losing the two s electrons and its f7 configuration accounts for the formation of this ion.
However, Eu2+ is a strong reducing agent changing to the common +3 state. Similarly Yb2+ which has f 14 configuration is a reductant.
TbIV has half-filled f-orbitals and is an oxidant. The behaviour of samarium is very much like europium, exhibiting both +2 and +3 oxidation states.
General Characteristics :
All the lanthanides are silvery white soft metals and tarnish rapidly in air.
The hardness increases with increasing atomic number, samarium being steel hard.
Their melting points range between 1000 to1200K but samarium melts at 1623 K.
They have typical metallic structure and are good conductors of heat and electricity. Density and other properties change smoothly except for Eu and Yb and occasionally for Sm and Tm.
Many trivalent lanthanide ions are coloured both in the solid state and in aqueous solutions. Colour of these ions may be attributed to the presence of f electrons. Neither La3+ nor Lu3+ ion shows any colour but the rest do so. However, absorption bands are narrow, probably because of the excitation within f level.
The lanthanide ions other than the f°type (La3+ and Ce4+) and the f14 type (Yb2+ and Lu3+) are all paramagnetic. The paramagnetism rises to maximum in neodymium.
The first ionisation enthalpies of the lanthanides are around 600 kJ mol–1, the second about 1200 kJ mol–1 comparable with those of calcium.
A detailed discussion of the variation of the third ionisation enthalpies indicates that the exchange enthalpy consideration (as in 3d orbitals of the first transition series), appear to impart a certain degree of stability to empty, half-filled and completely filled orbitals f level. This is indicated from the abnormally low value of the third ionization enthalpy of lanthanum, gadolinium and lutetium.
In their chemical behaviour, in general, the earlier members of the series are quite reactive similar to calcium but, with increasing atomic number, they behave more like aluminium.
Values for E– for the half-reaction:
Ln3+ (aq) + 3e−→ Ln(s) are in the range of –2.2 to –2.4 V except for Eu for which the value is – 2.0 V. This is, of course, a small variation.
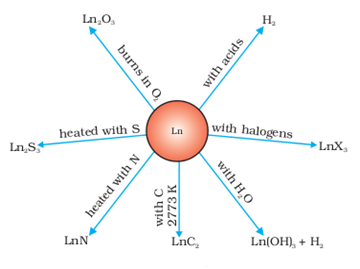
The metals combine with hydrogen when gently heated in the gas.
The carbides, Ln3C, Ln2C3 and LnC2 are formed when the metals are heated with carbon.
They liberate hydrogen from dilute acids and burn in halogens to form halides.
They form oxides M2O3 and hydroxides M(OH)3. The hydroxides are definite compounds, not just hydrated oxides.
They are basic like alkaline earth metal oxides and hydroxides.
The best single use of the lanthanides is for the production of alloy steels for plates and pipes. A well known alloy is mischmetall which consists of a lanthanide metal (~ 95%) and iron (~ 5%) and traces of S, C, Ca and Al. A good deal of mischmetall is used in Mg-based alloy to produce bullets, shell and lighter flint. Mixed oxides of lanthanides are employed as catalysts in petroleum cracking. Some individual Ln oxides are used as phosphors in television screens and similar fluorescing surfaces.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
