- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Potassium Permanganatic (KMnO4)
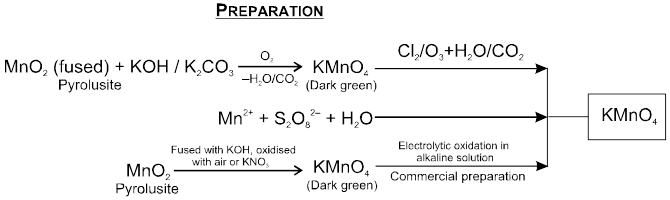
Physical Properties:
Purple coloured crystalline compound.
Moderately soluble in water at room temperature.
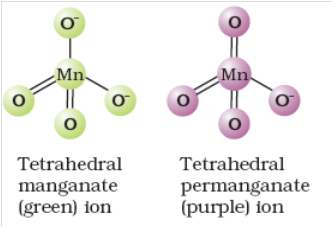
Structure of manganate and permanganate ion.
Chemical Properties
(i) Heating effect
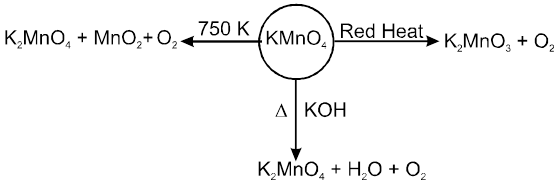
MnO42– in dilute alkaline, water and acidic solutions is unstable and disproportionates to give MnO4– and MnO2.
3MnO42– + 4H+ ¾®2MnO4– + MnO2 ¯ + 2H2O
3MnO42– + 2H2O ¾® 2MnO4– + MnO2 ¯ + 4OH–
or
3MnO42– + 3H2O ¾® 2MnO4– + MnO (OH)2 ¯+ 4OH–
On heating in current of H2 , solid KMnO4 gives MnO
2KMnO4 + 5H2 ![]() 2KOH + 2MnO + 4H2O
2KOH + 2MnO + 4H2O
(ii) On treatment with concentrated H2SO4 (KMnO4 is taken in excess), it forms manganese heptoxide via permanganyl sulphate which decomposes explosively on heating.
2KMnO4 + 3H2SO4 ¾® 2KHSO4 + (MnO3)2SO4 + 2H2O
(MnO3)2SO4 + H2O ¾® Mn2O7 + H2SO4
Mn2O7 ¾® 2MnO2 + ![]() O2
O2
KMnO4 + 3H2SO4 (conc.) ¾® K+ + MnO3+ (green) + 3HSO4– + H3O+.
(iii) Potassium permanganate is a powerful oxidising agent.
Potassium permanganate acts as an oxidising agent in alkaline, neutral or acidic solutions.
A mixture of sulphur, charcoal and KMnO4 forms an explosive powder. A mixture of oxalic acid and KMnO4 catches fire spontaneous after a few seconds. The same thing happens when glycerine is poured over powdered KMnO4.
In alkaline & neutral medium :
In strongly alkaline medium KMnO4 is reduced to manganate.
2KMnO4 + 2KOH (conc.) ¾® 2K2 MnO4 + H2O + [O]
or e– + MnO4– ¾® MnO42–
if solution is dilute then K2MnO4 is converted in to MnO2 which appears as a brownish precipitate.
2K2MnO4 + 2H2O ¾® 2MnO2 + 4KOH + 2[O]
2e– + 2H2O + MnO42– ¾® MnO2 + 4OH–
This type of behaviour is shown by KMnO4 itself in neutral medium.
3e– + 2H2O + MnO4– ¾® MnO2 + 4OH–
In alkaline or neutral medium KMnO4 shows following oxidising properties.
(a) It oxidises ethene to glycol.
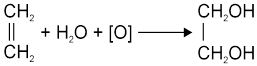
In alkaline medium KMnO4 solution is also known as Bayer’s reagent (1% alkaline KMnO4 solution).
(b) It oxidises iodide into iodate.
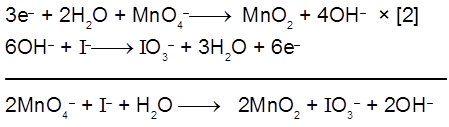
(c) H2S is oxidised into sulphur :
2MnO4– + 3H2S ¾® 2MnO2 + 2OH– +2H2O + 3S
(d) Manganous salt is oxidised to MnO2 ; the presence of zinc sulphate or zinc oxide catalyses the oxidation :
2MnO4– + 3Mn2+ + 2H2O ¾® 5MnO2 + 4H+
(e) In neutral/ faintly alkaline solution thiosulphate is quantitatively oxidised to sulphate.
8MnO4– + 3S2O32– + H2O ¾® 8MnO2 + 6SO42– + 2OH–
(f) In the presence of sodium hydroxide, sodium sulphite is oxidised in to sodium sulphate.
2MnO4– + 3SO32– + 3H2O ¾® 2MnO(OH)2¯+ 3SO42– + 2OH–.
In acidic medium (in presence of dilute H2SO4) :
Manganous sulphate is formed. The solution becomes colourless.
2KMnO4 + 3H2SO4 ¾® K2SO4 + 2MnSO4 + 3H2O + 5[O]
or MnO4¯ + 8H+ + 5e– ¾® Mn2+ + 4H2O
This medium is used in quantitative (volumetric) estimations. The equivalent mass of KMnO4 in acidic medium is = 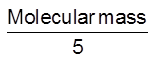 .
.
(a) Ferrous salts are oxidised to ferric salts.
MnO4¯ + 5Fe2+ + 8H+ ¾® 5Fe3+ + Mn2+ + 4H2O
(b) Iodine is evolved from potassium iodide.
2MnO4¯ + 10I¯ + 16H+ ¾® 2Mn2+ + 5I2 + 8H2O
(c) H2S is oxidised to sulphur :
2MnO4– + 16H++ 5S2– ¾® 2Mn2+ + 8H2O + 5S
(d) SO2 is oxidised to H2SO4 :
2MnO4– + 5SO2 + 2H2O ¾® 5SO42– + 2Mn2+ + 4H+
(e) Nitrites are oxidised to nitrates :
2MnO4– + 5NO2– + 6H+ ¾® 2Mn2+ + 3H2O + 5NO3–
(f) Oxalic acid is oxidised to CO2 :
This reaction is slow at room temperature, but is rapid at 60ºC.
Mn(II) ions produced catalyse the reaction; thus the reaction is autocatalytic.
2MnO4– + 16H+ + 5C2O42–![]() 2Mn2+ + 8H2O + 10CO2
2Mn2+ + 8H2O + 10CO2
(g) HCHO is oxidised to HCOOH
2MnO4– + 5HCHO + 6H+ ¾® 2Mn2+ + 5HCOOH + 3H2O.
(h) It oxidises hydrogen halides (HCl, HBr or HI) into X2 (halogen)
2MnO4– + 16H+ + 10X– ¾® 2Mn2+ + 8H2O + 5X2
(i) H2O2 is oxidised to O2.
2MnO4– + 6H+ + 5H2O2 ¾® 5O2 + Mn2+ + 8H2O.
Uses :It is used
- KMnO4 is used as an oxidising agent in laboratory and industry.
- Alkaline potassium permanganate is called Bayer's reagent. This reagent is used in organic chemistry for the test of unsaturation. KMnO4 is used in the manufacture of saccharin, benzoic acid, acetaldehyde etc.
- KMnO4 is used in qualitative analysis for detecting halides, sulphites, oxalates, etc.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
