- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
POTASSIUM DICHROMATE (K2Cr2O7)
Preparation :
The chromite ore is roasted with sodium carbonate in presence of air in a reverberatory furnace.
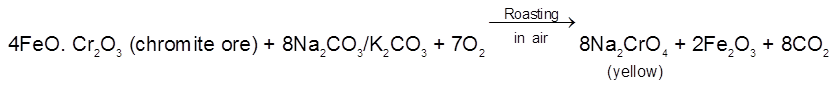
The roasted mass is extracted with water when Na2CrO4 goes into the solution leaving behind
insoluble Fe2O3. The solution is then treated with calculated amount of H2SO4.
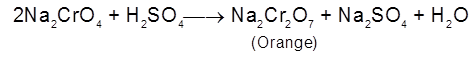
The solution is concentrated when less soluble Na2SO4 crystallises out. The solution is further concentrated when crystals of Na2Cr2O7 are obtained. Hot saturated solution of Na2Cr2O7 is then treated with KCl when orange red crystals of K2Cr2O7 are obtained on crystallisation. Sodium dichromate is more soluble than potassium dichromate.
Na2Cr2O7 + 2KCI ¾® K2Cr2O7 + 2 NaCl
K2Cr2O7 is preferred over Na2Cr2O7 as a primary standard in volumetric estimation because Na2Cr2O7 is
hygroscopic in nature but K2Cr2O7 is not.
Properties
(a) Physical :
It is orange-red coloured crystalline compound. It is moderately soluble in cold water but freely soluble in hot water. It melts at 398°C.
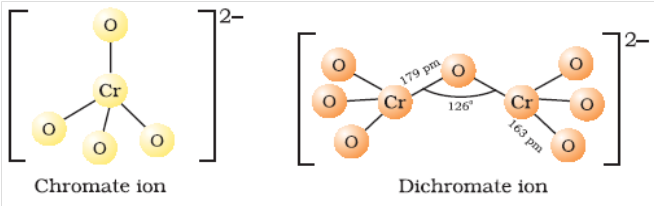
Structure of Chromate and Dichromate ion
(b) Chemical :
(i) Effect of heating :
On heating strongly, it decomposes liberating oxygen.
2K2Cr2O7 ¾® 2K2CrO4 + Cr2O3 + ![]() O2
O2
On heating with alkalies, it is converted to chromate, i.e., the colour changes from orange to yellow. On acidifying, yellow colour again changes to orange.
K2Cr2O7 + 2KOH ¾® 2K2CrO4 + H2O
Cr2O72-(Orange) + 2OH¯ ¾® 2CrO42- (Yellow)+ H2O
2CrO(Yellow) + 2H+ ¾® Cr2O72- (Orange) + H2O
Thus CrO42– and Cr2O72– exist in equilibrium and are interconvertable by altering the pH of solution.
2CrO42- + 2H+![]() 2HCrO4–
2HCrO4–![]() Cr2O72- + H2O
Cr2O72- + H2O
In alkaline solution, chromate ions are present while in acidic solution, dichromate ions are present.
(ii) K2Cr2O7 + 2H2SO4 (conc. & cold) ¾® 2CrO3¯ (bright orange/red) + 2KHSO4 + H2O
2K2Cr2O7 + 8H2SO4 (conc. & Hot) ¾® 2K2SO4 + 8H2O + 2Cr2(SO4)3 + 3O2
(iii) Acidified K2Cr2O7 solution reacts with H2O2 to give a deep blue solution due to the formation of CrO5.
Cr2O72– + 2H+ + 4H2O2 ¾® 2CrO5 + 5H2O
Blue colour in aqueous solution fades away slowly due to the decomposition of CrO5 to Cr3+ ions and oxygen. In less acidic solution K2Cr2O7 and H2O2 give salt which is violet coloured and diamagnetic due to the formation of [CrO(O2)(OH)]–.
(iv)Potassium dichromate reacts with hydrochloric acid and evolves chlorine gas.
K2Cr2O7 + 14HCl ¾® 2KCl + 2CrCl3 + 7H2O + 3Cl2
(v) It acts as a powerful oxidising agent in acidic medium (dilute H2SO4)
Cr2O72- + 14H+ + 6e– ¾® 2Cr+3 + 7H2O. (Eº = 1.33 V)
The oxidation state of Cr changes from + 6 to +3.
(a) Iodine is liberated from potassium iodide :
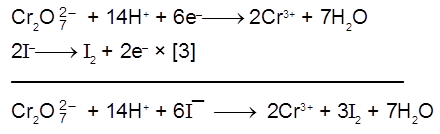
(b) Ferrous salts are oxidised to ferric salts :
6Fe2+ + Cr2O72- + 14H+ ¾® 6Fe3+ + 2Cr3+ + 7H2O
(c) Sulphites are oxidised to sulphates :
Cr2O72- + 3SO32- + 8H+¾® 3SO42- + 2Cr3+ + 4H2O
(d) H2S is oxidised to sulphur :
Cr2O72- + 3H2S + 8H+ ¾® 2Cr3+ + 7H2O + 3S
(e) SO2 is oxidised to H2SO4 :
Cr2O72– + 3SO2 + 2H+ ¾® 2Cr3+ + 3SO42– + H2O ;
Chrome alum is obtained when acidified K2Cr2O7 solution is saturated with SO2.
K2Cr2O7 + H2SO4 + 3SO2 + 23H2O ![]() K2SO4 . Cr2(SO4)3 . 24H2O
K2SO4 . Cr2(SO4)3 . 24H2O
(f) It oxidises ethylalcohol to acetaldehyde and acetaldehyde to acetic acid
C2H5OH ![]() CH3CHO
CH3CHO ![]() CH3COOH
CH3COOH
ethyl alcohol acetaldehyde acetic acid
(g) It also oxidises nitrites to nitrates, arsenites to arsenates, HBr to Br2. HI to I2, etc.
(h) K2Cr2O7 + 2C (charcoal) ![]() Cr2O3 + K2CO3 + CO ↑
Cr2O3 + K2CO3 + CO ↑
(vi) Chromyl chloride test :
4Cl– + Cr2O72– + 6H+ ¾® 2CrO2Cl2 ↑ (deep red) + 3H2O
CrO2Cl2 + 4OH– ¾® CrO42– (yellow) + 2Cl– + 2H2O
CrO42– (yellow) + Pb2+ ¾® PbCrO4 ↓ (yellow)
(vii) Cr2O72– (concentrated solution) + 2Ag+ ¾® Ag2Cr2O7↓ (reddish brown)
Ag2Cr2O7 ↓ + H2O ![]() Ag2CrO4 ↓+ CrO42– + 2H+ .
Ag2CrO4 ↓+ CrO42– + 2H+ .
(viii) Cr2O72– + Ba2+ + H2O ![]() 2BaCrO4 ↓+ 2H+
2BaCrO4 ↓+ 2H+
As strong acid is produced, the precipitation is only partial. But if NaOH or CH3COONa is added, precipitate becomes quantitative.
Uses : It is used :
(i) as a volumetric reagent in the estimation of reducing agents such as oxalic acid, ferrous ions, iodide ions, etc. It is used as a primary standard.
(ii) for the preparation of several chromium compounds such as chrome alum, chrome yellow, chrome red, zinc yellow, etc.
(iii) in dyeing, chrome tanning, calico printing, photography etc.
(iv) as a cleansing agent for glass ware in the form of chromic acid.
in leather industry and as an oxidant for preparation of azo compounds.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
