- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Catalysts
Berzillus in 1835 used the word catalyst first time for some substance which alter rate of chemical reaction and themselves remain chemically and quantitatively unchanged after the reaction and the phenomenon is known as catalysis.
Eg : Potassium chlorate when heated at 653K to 873K, it gives O2, When MnO2 is used in this reaction the O2 is quickly at the low temperature hence MnO2 is a catalyst
2KCIO3 ® 2KCI + 3O2
Homogeneous Catalysis : When catalysts and reactants are in same phase then the process is said to be homogeneous catalysis and
Eg :
(i) ![]()
(ii) ![]()
(iii) 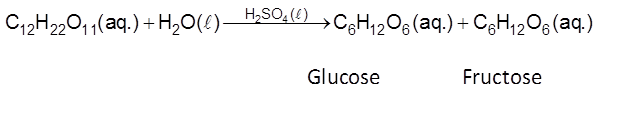
Heterogenous Catalysis : When catalysts and reactants are in different phases, then process a know as heterogenous catalysis and catalyst is called heterogeneous catalyst
Eg :
(i) ![]()
(ii) ![]()
(iii) ![]()
(iv) Vegetable oils![]() Vegetable ghee (s).
Vegetable ghee (s).
Types of Catalysis
(a) Positive Catalysis : A substance which increase the rate of chemical reaction is called positive catalyst and this process called positive catalysis.
(i) 
(ii) 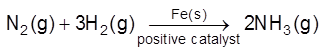
(b) Negative Catalysis : A substance which decrease the rate of chemical reaction is called negative catalyst and this process called negative catalysis.
Eg :
(i) 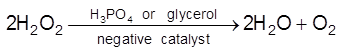
(ii) Decomposition of chloroform reduces in the presence of 1% ethyl alcohol.
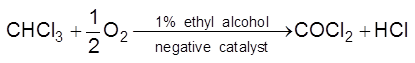
(iii) T.E.L. is used as negative catalyst in petrol which reduce knocking.
(c) Auto Catalysis : When one of the reaction product behave as catalyst for that reaction and increase the rate of reaction then the phenomenon is called autocatalysis.
“Auto catalytic reactions are slow in the beginning but become increasingly rapid as the reaction proceeds.
Eg :
(i) ![]()
(ii)
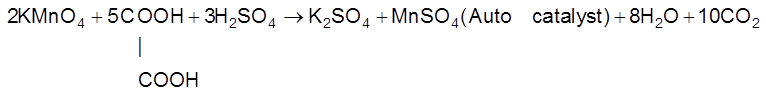
(d) Induced Catalyst : When one reaction catalyse an other reaction than the phenomenon is called induced catalysis and that reaction is called induced catalyst.
Promoters/Activators : Substance which themselves are not catalyst but its presence can increase the catalytic activity of catalyst. A promoters increase the number of active sites on the surface Eg :
(i) 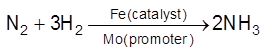
(ii) Vegetable Oil + H2 ![]() Vegetable ghee.
Vegetable ghee.
(iii) ![]()
Catalytic Poisons/ Anti catalysts/ Catalyst Inhibitor : Substance which themselves are not catalyst but whose presence decrease the activity of the catalyst. Poisoning is due to preferential adsorption of poison on the surface of the catalyst.
(i) 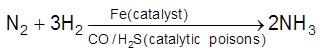
(ii) 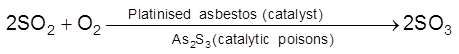
(iii)Rosunmund Reactions : ![]()
Characteristics of Catalysis :
(i) A Catalyst remains unchanged in mass and chemical compositions at the end of reactions. However its physical state can be change. Eg :
Granular MnO2 during decomposition of KClO3 is left as powder at the end of the reaction.
(ii) Finely devided state of catalyst is more efficient for the reactions because surface area increases and more adsorption take place.
(iii)A small amount of catalyst is generally sufficient to catalyse almost unlimited reaction but in some cases the rate of reaction depends on amount of catalyst.
Exception :
(a) In Friedal Craft reaction more amount of catalyst is required.
(b) Hydrolysis of ester in acidic and alkaline medium its rate of reaction is proportional to concentration of H+ or OH– ions.
(iv) A catalyst cannot initiate reaction. But some times the activation energy is so large that practically a reaction may not start until a catalyst lowers the activation energy significantly. For example, mixture of hydrogen and oxygen do not react at room temperature but the reaction occurs very rapid in presence of Pt black.
H₂ + O2 ![]() No reaction
No reaction
H₂ + O2 ![]() H2O.
H2O.
(v) Catalyst are generally specific in nature. A substance which act as a catalyst in a particular reaction, fails to catalyse other reaction.
(vi) Catalyst cannot change equilibrium state but it help to attain equilibrium quickly.
(vii) A catalyst does not change the enthalpy, entropy and free energy of a reaction.
(viii) Optimum temperature : There is a particular temperature at which the efficiency of a catalyst a maximum this temperature is known as optimum temperature. On either side the optimum temperature, the activity of catalyst decreases.
Adsorption Theory of Heterogeneous Catalyst : This theory explain the mechanism of heterogeneous catalyst. This theory is combination of two theory, intermediate compound formation theory and the old adsorption theory, the catalytic activity is localised on the surface on the catalyst. The mechanism involve 5 steps.
(i) Diffusion of reactant to the surface of the catalyst.
(ii) Adsorption of reactant molecules on the surface of the catalyst.
(iii) Formation of activated intermediate.
(iv) Formation of reactions product on the catalyst surface.
(v) Diffusion of reactions product from the catalyst surface or desorption.
Examples :
Let us consider addition of H2 gas to ethelene in presence of Ni catalyst, the reaction take places as follows.
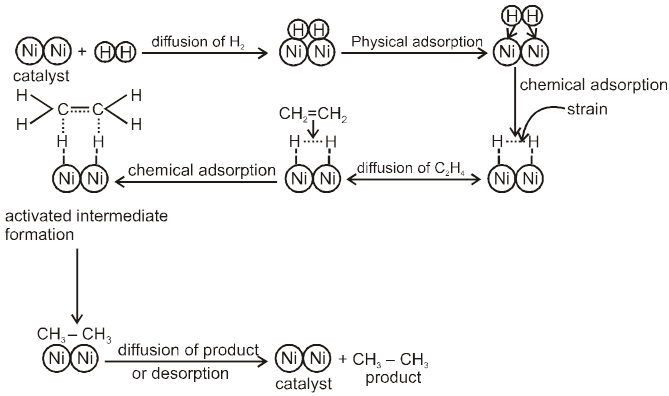
Factors Supporting Theory :
(i) This theory explain the role of active centre, more free valency which provide the more space for the more adsorption and concentration increases as a result increase in rate of reaction.
(ii) Rough surface has more active and pores there will be more free valency so more will be rate of reaction.
(iii) The theory explain centre action of promoters which occupied interstial void as a result surface area for the adsorption increases therefore rate of reaction increases.
(iv)The theory explain of function of poisons or inhibitors. In poisoning preferential adsorption of poisons take place on the catalyst, surface area for the adsorption on the catalyst decrease hence rate of reaction decreases.
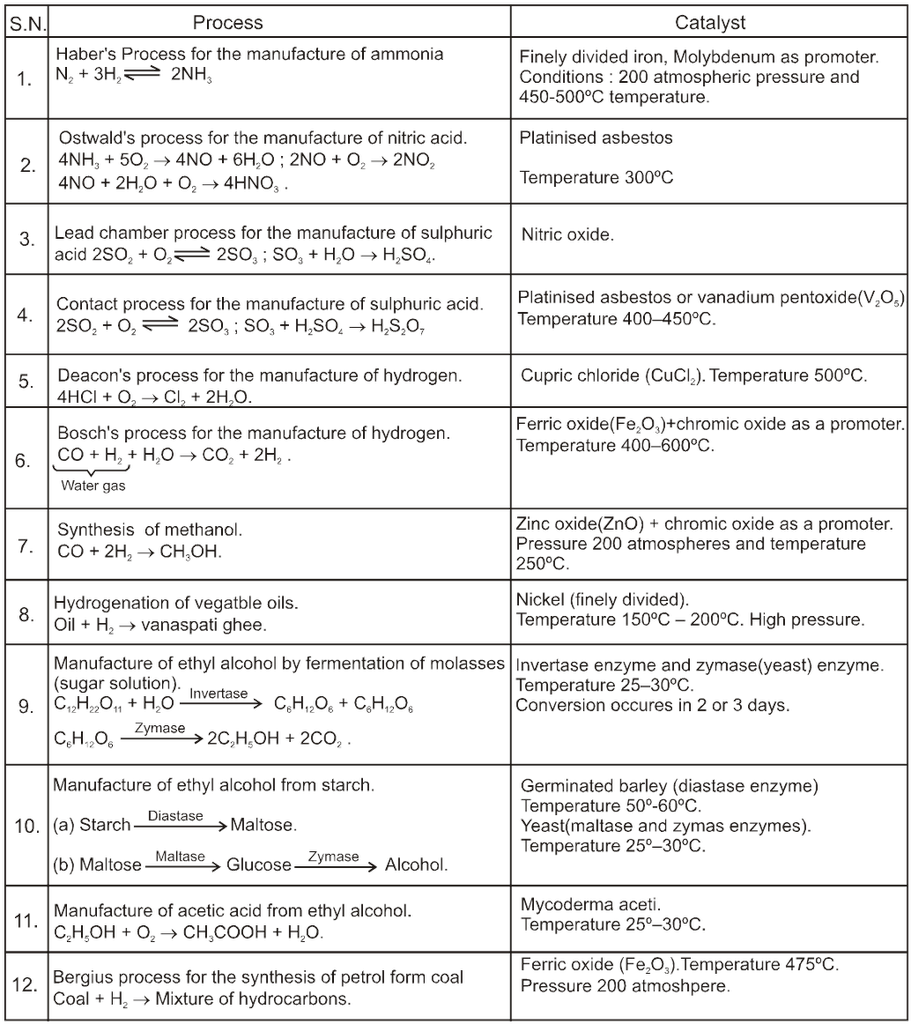
Some Industrial Catalytic reactions

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
