- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
OXY-ACIDS OF Halogens
Fluorine forms only one oxoacid, HOF due to high electronegativity and small size. Other halogens form a number of oxoacids which are stable only in aqueous solutions or in the form of their salts. They can not be isolated in pure form.
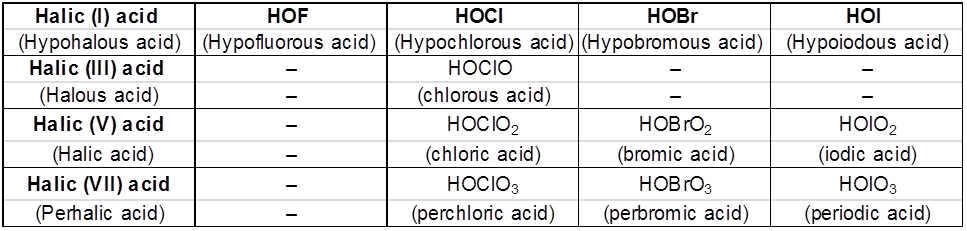
Some important order
(a) Acid strength
(i) HI > HBr > HCl > HF
(ii) HOCl > HOBr > HOI
(iii) HClO4 > HClO3 >HClO2 > HClO
(b) Oxidising powder
(i) F2 > Cl2 > Br2 > I2
(ii) BrO4– > IO4– > ClO4– (According to electrode potential)
(c) Order of disproportionations
3 XO– ® 2X– + XO3– (hypohalite ion) ;
IO– > BrO– > ClO–

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
