- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Phosphorus Halides
Phsophorus forms two types of halides , PX3 [X = F , Cl , Br, I] and PX5 [X = F , Cl , Br]
(a) Phosphorus Trichloride :
Preparation :
(i) .It is obtained by passing dry chlorine over heated white phosphorus
P4 + 6 Cl2 ® 4 PCl3
(ii) It is also obtained by the action of thionyl chloride with white phosphorus.
P4 + 8 SOCl2 ® 4 PCl3 + 4 SO2 + 2 S2Cl2
Properties :
(i) It is a colourless oily liquid and hydrolyses in the presence of moisture.
PCl3 + 3 H2O ® H3PO3 + 3 HCl
(ii) It reacts with organic compounds containing – OH group such as CH3COOH , C2H5OH.
3 CH3COOH + PCl3 ® 3 CH3COCl + H3PO3
3 C2H5OH + PCl3 ® 3 C2H5Cl +H3PO3
(b) Phosphorus pentachloride :
Preparation :
Phosphorus pentachloride is prepared by the reaction of white phosphorus with excess of dry chlorine.
P4 + 10 Cl2 ® 4 PCl5
It can also be prepared by the action of SO2Cl2 on phosphorus.
P4 + 10 SO2Cl2 ® 4 PCl5 + 10 SO2
Properties :
(i) PCl5 is a yellowish white powder and in moist air , it hydrolyses to POCl3 and finally gets converted to phosphoric acid.
PCl5 + H2O ® POCl3 + 2 HCl
POCl3 + 3 H2O ® H3PO4 + 3 HCl
(ii) When heated it sublimes but decomposes on stronger heating.
PCl5 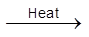 PCl3 + Cl2
PCl3 + Cl2
(iii) It reacts with organic compounds containing – OH group converting them to chloro derivatives.
C2H5OH + PCl5 ® C2H5Cl + POCl3 + HCl
CH3COOH + PCl5 ® CH3COCl + POCl3 + HCl
(iv) PCl5 on heating with finely divided metals give corresponding chlorides.
2 Ag + PCl5 ® 2 AgCl + PCl3
Sn + 2 PCl5 ® SnCl4 + 2 PCl3
It is used in the synthesis of some organic compounds , e.g., C2H5Cl , CH3COCl.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
