- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chapter 8
p-block elements
(halogens family and noble gases)
Introduction :
Group 13 to 18 of the periodic table of elements constitute the p–block. The p–block contains metals, metalloids as well as non–metals.
The p–block elements have general valence shell electronic configuration ns2 np1–6.
The first member of each group from 13–17 of the p–block elements differ in many respects from the other members of their respective groups because of small size, high electronegativity and absence of d–orbitals.
The first member of a group also has greater ability to form pp–pp multiple bonds to itself (e.g. C=C, C=C, N º N) and to element of second row (e.g C=O, C=N, CºN, N=O) compared to the other members of the same group.
The highest oxidation of p–block element is equal to the group number minus 10. Moving down the group, the oxidation state two less than the highest group oxidation state becomes more stable in groups 13 to 16 due to inert pair effect (reluctance of s-subshell electrons to participate in chemical bonding)
Group 17 Elements : The Halogen Family
Electronic Configuration : All these elements have seven electrons in their outermost shell (ns2 np5) which is one electron short of the next noble gas.
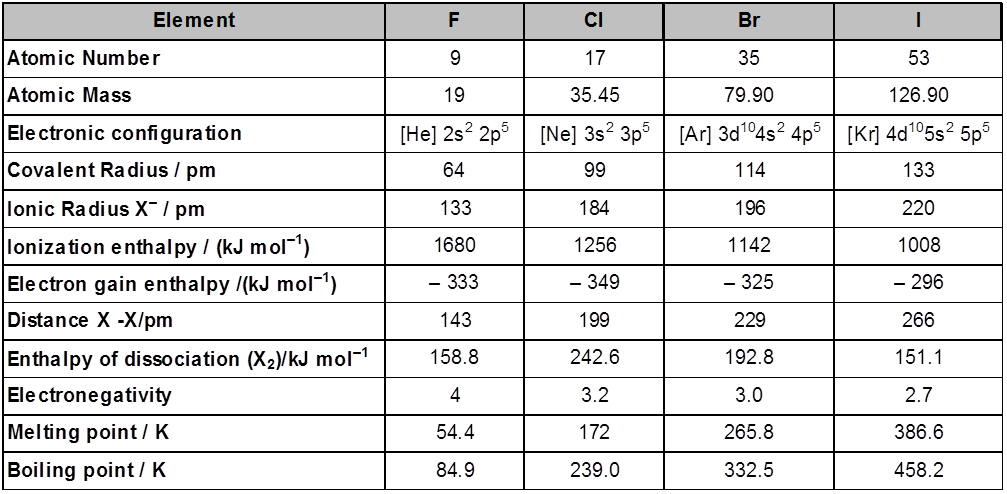
Atomic and Ionic Radii : The halogens have the smallest atomic radii in their respective periods due to maximum effective nuclear charge . Atomic and ionic radii increase from fluorine to iodine due to increasing number of quantum shells.
Ionisation Enthalpy : They have little tendency to lose electron. Thus they have very high ionisation enthalpy. Due to increase in atomic size, ionisation enthalpy decreases down the group.
Electron Gain Enthalpy : Halogen have maximum negative electron gain enthalpy in the corresponding period. This is due to the fact that the atoms of these elements have only one electron less than stable noble gas configurations. Electron gain enthalpy of the elements of the group becomes less negative down the group. However, the negative electron gain enthalpy of fluorine is less than that of chlorine. It is due to small size of fluorine atom. As a result, there are strong interelectronic repulsions in the relatively small 2p orbitals of fluorine and thus, the extra electron (incoming) does not experience much attraction.
Electronegativity : They have very high electronegativity. The electronegativity decreases down the group. Fluorine is the most electronegative element in the periodic table
Physical Properties : Fluorine and chlorine are gases, bromine is a liquid whereas iodine is a solid. Their melting and boiling points steadily increase with atomic number. All halogens are coloured. This is due to absorption of radiations in visible region which results in the excitation of outer electrons to higher energy level. By absorbing different quanta of radiation, they display different colours. For example, F2, has yellow, Cl2, greenish yellow, Br2, red and I2, violet colour. Fluorine and chlorine react with water. Bromine and iodine are only sparingly soluble in water. But are soluble in organic solvents such as chloroform, carbon tetrachloride, carbon disulphide and hydrocarbons to give coloured solutions. Except the smaller enthalpy of dissociation of F2 compared to that of Cl2. The X-X bond disassociation enthalpies from chlorine onwards show the expected trend : Cl – Cl > Br – Br > F – F > I – I. The reason for the smaller enthalpy of dissociation of F2 is the relatively larger electrons-electrons repulsion among the lone pairs in F2 molecule where they are much closer to each other than in case of Cl2.
Atomic & physical properties
Chemical Properties
Oxidation states and trends in chemical reactivity
All the halogens exhibit –1 oxidation state. However, chlorine, bromine and iodine exhibit + 1, + 3, + 5 and + 7 oxidation states also. The higher oxidation states of chlorine, bromine and iodine are realised mainly when the halogens are in combination with the small and highly electronegative fluorine and oxygen atoms e.g., in interhalogens, oxides and oxoacids.
The fluorine atom has no d orbitals in its valence shell and therefore cannot expand its octet. Being the most electronegative, it exhibits only – 1 oxidation state.
All the halogens are highly reactive. They react with metals and non-metals to form halides. The reactivity of the halogens decreases down the group.
The ready acceptance of an electron is the reason for the strong oxidising nature of halogens. F2 is the strongest oxidising halogen and it oxidises other halide ions in solution or even in the solid phase. The decreasing oxidising ability of the halogens in aqueous solution down the group is evident from their standard electrode potentials. Fluorine oxidises water to oxygen whereas chlorine and bromine react with water to form corresponding hydrohalic and hypohalous acids. The reactions of iodine with water is non- spontaneous . I– can be oxidised by oxygen in acidic medium; just the reverse of the reaction observed with fluorine.
2F2(g) + 2H2O(l) ® 4H+ (aq) + 4F– (aq) + O2(g)
X2(g) + H2O (l) ® HX(aq) + HOX (aq) ; (where X = Cl or Br)
4I– (aq) + 4H+ (aq) + O2(g) ® 2 I2 (s) + 2H2O (l)
Standard Reduction Potential (SRP)
X2 + 2e– ® 2X–
F2 + 2e– ® 2F– [E° = + 2.87 V ]
Cl2 + 2e– ® 2Cl– [E° = + 1.36 V]
Br2 + 2e – ® 2Br – [E° = + 1.09 V ]
I2 + 2e– ® 2I– [E° = + 0.54 V]
More the value of the SRP, more powerful is the oxidising agent. Hence the order of oxidising power is
F2 > Cl2 > Br2 > I2
Since SRP is the highest for F2 (among all elements of periodic table), it is a strongest oxidising agent.
Hydration energy of X–
Smaller the ion, higher is the hydration energy.
F – Cl – Br – I–
515 381 347 305 in kJ/mol
Anomalous behaviour of fluorine
The anomalous behaviour of fluorine is due to its small size, highest electronegativity, low F- F bond dissociation enthalpy, and non availability of d orbitals in valence shell. Most of the reactions of fluorine are exothermic (due to the small and strong bond formed by it with other elements). It forms only one oxoacid while other halogens form a number of oxoacids. Hydrogen fluoride is liquid (b.p. 293 K) due to strong hydrogen bonding. Other hydrogen halides are gases.
(i) Reactivity towards hydrogen : They all react with hydrogen to give hydrogen halides but affinity for hydrogen decreases from fluorine to iodine with increasing atomic number. They dissolve in water to form hydrohalic acids. The acidic strength of these acids increases in the order : HF < HCl < HBr < HI. The stability of these halides decreases down the group due to decrease in bond (H–X) dissociation enthalpy in the order :
H – F > H – Cl > H –Br > H – I .
(ii) Reactivity towards oxygen : Halogens form many oxides with oxygen but most of them are unstable. Fluorine forms two oxides OF2 and O2F2. However, only OF2 is the thermally stable at 298 K. These oxide are essentially oxygen fluorides because of the higher electronegativity of flurorine than oxygen . Both are strong fluorinating agents. O2F2 oxidises plutonium to PuF6 and the reaction is used in removing plutonium as PuF6 from spent nuclear fuel.
Chlorine, bromine and iodine form oxides in which the oxidation states of these halogen vary from + 1 to + 7. A combination of kinetic and thermodynamic factors lead to the generally decreasing order of stability of oxides formed by halogens, I > Cl > Br. The higher oxides of halogens tend to be more stable than the lower ones.
Chlorine oxides, Cl2O, ClO2, Cl2O6 and Cl2O7 are highly reactive oxidising agents and tend to explode. ClO2 is used as a bleaching agent for paper pulp and textiles and in water treatment.
The bromine oxides, Br2O, BrO2, BrO3 are the least stable halogen oxides and exist only at low temperature. They are very powerful oxidising agents.
The iodine oxides, I2O4, I2O5, I2O7 are insoluble solids and decompose on heating. I2O5 is very good oxidising agent and is used in the estimation of carbon monoxide.
(iii) Reactivity towards metals : Halogen react with metals to form metal halides. For e.g., bromine reacts with magnesium to give magnesium bromide.
(iv) Reactivity of halogen towards other halogens : Halogens combine amongst themselves to form a number of compounds known as interhalogen of the types AB, AB3, AB5 and AB7 where A is a larger size halogen and B is smaller size halogen.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
