1.Group 17 elements
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chapter 8
p-block elements
(halogens family and noble gases)
Introduction :
Group 13 to 18 of the periodic table of elements constitute the p–block. The p–block contains metals, metalloids as well as non–metals.
The p–block elements have general valence shell electronic configuration ns2 np1–6.
The first member of each group from 13–17 of the p–block elements differ in many respects from the other members of their respective groups because of small size, high electronegativity and absence of d–orbitals.
The first member of a group also has greater ability to form pp–pp multiple bonds to itself (e.g. C=C, C=C, N º N) and to element of second row (e.g C=O, C=N, CºN, N=O) compared to the other members of the same group.
The highest oxidation of p–block element is equal to the group number minus 10. Moving down the group, the oxidation state two less than the highest group oxidation state becomes more stable in groups 13 to 16 due to inert pair effect (reluctance of s-subshell electrons to participate in chemical bonding)
Group 17 Elements : The Halogen Family
Electronic Configuration : All these elements have seven electrons in their outermost shell (ns2 np5) which is one electron short of the next noble gas.
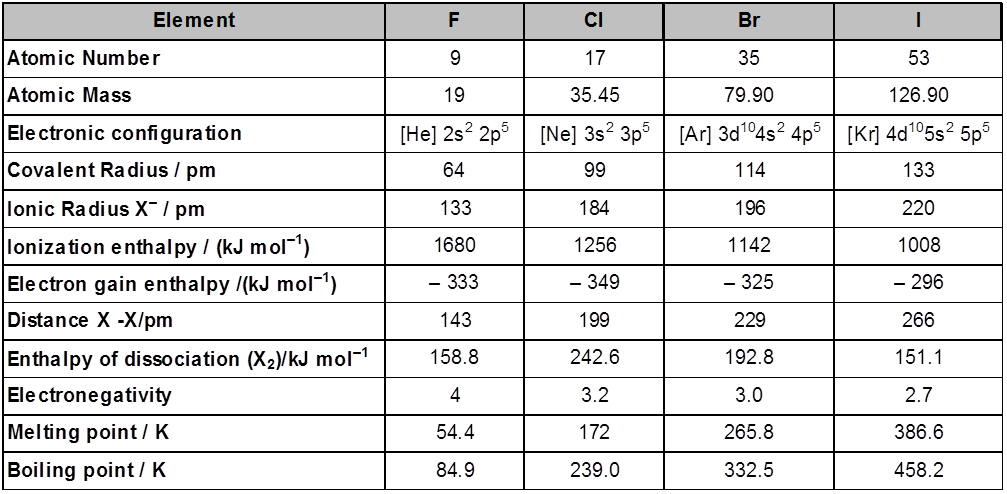
Atomic and Ionic Radii : The halogens have the smallest atomic radii in their respective periods due to maximum effective nuclear charge . Atomic and ionic radii increase from fluorine to iodine due to increasing number of quantum shells.
Ionisation Enthalpy : They have little tendency to lose electron. Thus they have very high ionisation enthalpy. Due to increase in atomic size, ionisation enthalpy decreases down the group.
Electron Gain Enthalpy : Halogen have maximum negative electron gain enthalpy in the corresponding period. This is due to the fact that the atoms of these elements have only one electron less than stable noble gas configurations. Electron gain enthalpy of the elements of the group becomes less negative down the group. However, the negative electron gain enthalpy of fluorine is less than that of chlorine. It is due to small size of fluorine atom. As a result, there are strong interelectronic repulsions in the relatively small 2p orbitals of fluorine and thus, the extra electron (incoming) does not experience much attraction.
Electronegativity : They have very high electronegativity. The electronegativity decreases down the group. Fluorine is the most electronegative element in the periodic table
Physical Properties : Fluorine and chlorine are gases, bromine is a liquid whereas iodine is a solid. Their melting and boiling points steadily increase with atomic number. All halogens are coloured. This is due to absorption of radiations in visible region which results in the excitation of outer electrons to higher energy level. By absorbing different quanta of radiation, they display different colours. For example, F2, has yellow, Cl2, greenish yellow, Br2, red and I2, violet colour. Fluorine and chlorine react with water. Bromine and iodine are only sparingly soluble in water. But are soluble in organic solvents such as chloroform, carbon tetrachloride, carbon disulphide and hydrocarbons to give coloured solutions. Except the smaller enthalpy of dissociation of F2 compared to that of Cl2. The X-X bond disassociation enthalpies from chlorine onwards show the expected trend : Cl – Cl > Br – Br > F – F > I – I. The reason for the smaller enthalpy of dissociation of F2 is the relatively larger electrons-electrons repulsion among the lone pairs in F2 molecule where they are much closer to each other than in case of Cl2.
Atomic & physical properties
Chemical Properties
Oxidation states and trends in chemical reactivity
All the halogens exhibit –1 oxidation state. However, chlorine, bromine and iodine exhibit + 1, + 3, + 5 and + 7 oxidation states also. The higher oxidation states of chlorine, bromine and iodine are realised mainly when the halogens are in combination with the small and highly electronegative fluorine and oxygen atoms e.g., in interhalogens, oxides and oxoacids.
The fluorine atom has no d orbitals in its valence shell and therefore cannot expand its octet. Being the most electronegative, it exhibits only – 1 oxidation state.
All the halogens are highly reactive. They react with metals and non-metals to form halides. The reactivity of the halogens decreases down the group.
The ready acceptance of an electron is the reason for the strong oxidising nature of halogens. F2 is the strongest oxidising halogen and it oxidises other halide ions in solution or even in the solid phase. The decreasing oxidising ability of the halogens in aqueous solution down the group is evident from their standard electrode potentials. Fluorine oxidises water to oxygen whereas chlorine and bromine react with water to form corresponding hydrohalic and hypohalous acids. The reactions of iodine with water is non- spontaneous . I– can be oxidised by oxygen in acidic medium; just the reverse of the reaction observed with fluorine.
2F2(g) + 2H2O(l) ® 4H+ (aq) + 4F– (aq) + O2(g)
X2(g) + H2O (l) ® HX(aq) + HOX (aq) ; (where X = Cl or Br)
4I– (aq) + 4H+ (aq) + O2(g) ® 2 I2 (s) + 2H2O (l)
Standard Reduction Potential (SRP)
X2 + 2e– ® 2X–
F2 + 2e– ® 2F– [E° = + 2.87 V ]
Cl2 + 2e– ® 2Cl– [E° = + 1.36 V]
Br2 + 2e – ® 2Br – [E° = + 1.09 V ]
I2 + 2e– ® 2I– [E° = + 0.54 V]
More the value of the SRP, more powerful is the oxidising agent. Hence the order of oxidising power is
F2 > Cl2 > Br2 > I2
Since SRP is the highest for F2 (among all elements of periodic table), it is a strongest oxidising agent.
Hydration energy of X–
Smaller the ion, higher is the hydration energy.
F – Cl – Br – I–
515 381 347 305 in kJ/mol
Anomalous behaviour of fluorine
The anomalous behaviour of fluorine is due to its small size, highest electronegativity, low F- F bond dissociation enthalpy, and non availability of d orbitals in valence shell. Most of the reactions of fluorine are exothermic (due to the small and strong bond formed by it with other elements). It forms only one oxoacid while other halogens form a number of oxoacids. Hydrogen fluoride is liquid (b.p. 293 K) due to strong hydrogen bonding. Other hydrogen halides are gases.
(i) Reactivity towards hydrogen : They all react with hydrogen to give hydrogen halides but affinity for hydrogen decreases from fluorine to iodine with increasing atomic number. They dissolve in water to form hydrohalic acids. The acidic strength of these acids increases in the order : HF < HCl < HBr < HI. The stability of these halides decreases down the group due to decrease in bond (H–X) dissociation enthalpy in the order :
H – F > H – Cl > H –Br > H – I .
(ii) Reactivity towards oxygen : Halogens form many oxides with oxygen but most of them are unstable. Fluorine forms two oxides OF2 and O2F2. However, only OF2 is the thermally stable at 298 K. These oxide are essentially oxygen fluorides because of the higher electronegativity of flurorine than oxygen . Both are strong fluorinating agents. O2F2 oxidises plutonium to PuF6 and the reaction is used in removing plutonium as PuF6 from spent nuclear fuel.
Chlorine, bromine and iodine form oxides in which the oxidation states of these halogen vary from + 1 to + 7. A combination of kinetic and thermodynamic factors lead to the generally decreasing order of stability of oxides formed by halogens, I > Cl > Br. The higher oxides of halogens tend to be more stable than the lower ones.
Chlorine oxides, Cl2O, ClO2, Cl2O6 and Cl2O7 are highly reactive oxidising agents and tend to explode. ClO2 is used as a bleaching agent for paper pulp and textiles and in water treatment.
The bromine oxides, Br2O, BrO2, BrO3 are the least stable halogen oxides and exist only at low temperature. They are very powerful oxidising agents.
The iodine oxides, I2O4, I2O5, I2O7 are insoluble solids and decompose on heating. I2O5 is very good oxidising agent and is used in the estimation of carbon monoxide.
(iii) Reactivity towards metals : Halogen react with metals to form metal halides. For e.g., bromine reacts with magnesium to give magnesium bromide.
(iv) Reactivity of halogen towards other halogens : Halogens combine amongst themselves to form a number of compounds known as interhalogen of the types AB, AB3, AB5 and AB7 where A is a larger size halogen and B is smaller size halogen.
2. Chlorine
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
CHLORINE (Cl2)
Preparation :
(i) By heating MnO2 with concentrated hydrocloric acid.
MnO2 + 4HCl ® MnCl2 + Cl2 + 2H2O
(ii) By heating chloride with concentrated H2SO4 in presence of MnO2.
4H+ + MnO2 + 2X– ® X2 + Mn+2 + 2H2O
(iii) 2KMnO4 + 16 HCl ® 2 KCl + 2 MnCl2 + 5 Cl2 + 8 H2O
(iv) Manufacture of chlorine :
(a) Deacon’s process : By oxidation of hydrogen chloride gas by atmospheric oxygen in the presence of CuCl2 (catalyst) at 723 K.
4 HCl + O2 ![]() 2 Cl2 + 2 H2O
2 Cl2 + 2 H2O
(b) Electrolytic process : Chlorine is obtained by the electrolysis of brine (concentrated NaCl solution). Chlorine is liberated at anode. It is obtained as a by–product in many chemical industries e.g.; in mfg. of sodium hydroxide.
NaX (aq) ® Na+ (aq) + X– (aq)
Anode : 2X– ® X2 + 2e–
Properties :
(i) It is a greenish–yellow gas with pungent and suffocating odour. It is about 2–5 times heavier than air. It can be liquefied into greenish–yellow liquid which boils at 239 K. It is soluble in water.
(ii) At low temperature it forms a hydrate with water having formula Cl2 . 8H2O which is infact a clathrate compound.
(iii) Reaction with metals :
2Al + 3Cl2 ® 2AlCl3
2Fe + 3Cl2 ® 2FeCl3
Reaction with non-metals :
P4 + 6Cl2 ® 4PCl3
S8 + 4Cl2 ® 4S2Cl2
(iv) Affinity for hydrogen : It reacts with compounds containing hydrogen and form HCl.
H2 + Cl2 ® 2HCl
H2S + Cl2 ® 2HCl + S
(v) Reaction with NaOH :
(a) 2 NaOH ( cold & dilute) + Cl2 ® NaCl + NaClO + H2O
(b) 6 NaOH (hot & concentrated) + 3 Cl2 ® 5 NaCl + NaClO3 + 3 H2O
(vi) Reaction with dry slaked lime, Ca(OH)2 : To give bleaching powder.
2 Ca(OH)2 + 2 Cl2 ® Ca(OCl)2 + CaCl2 + 2 H2O
The composition of bleaching powder is Ca(OCl)2.CaCl2.Ca(OH)2 .2H2O
(vii) Oxidising & bleaching properties :
Chlorine dissolves in water (Cl2 water is yellow) giving HCl (colourless) and HOCl (colourless). Hypochlorous acid (HOCl) so formed, gives nascent oxygen which is responsible for oxidising and bleaching properties of chlorine.
(a) It oxidises ferrous to ferric, sulphite to sulphate, sulphur dioxide to sulphuric acid and iodine to iodic acid.
2 FeSO4 + H2SO4 + Cl2 ® Fe2(SO4)3 + 2 HCl
Na2SO3 + Cl2 + H2O ® Na2SO4 + 2 HCl
SO2 + 2 H2O + Cl2 ® H2SO4 + 2 HCl
I2 + 6 H2O + 5 Cl2 ® 2 HIO3 + 10 HCl
(b) It is a powerful bleaching agent ; bleaching action is due to oxidation.
Cl2 + H2O ® 2 HCl + O
Coloured substance + O ® Colourless substance
It bleaches vegetable or organic matter in the presence of moisture. Bleaching effect of chlorine is permanent.
Note :
The bleaching action of SO2 is temporary because it takes place through reduction.
SO2 + 2 H2O ® H2 SO4 + 2 H
SO32– + Coloured material SO42– + Reduced colourless material.
Reduced Colourless material ![]() Coloured material.
Coloured material.
Uses : Cl2 is used
1. for bleaching wood pulp (required for the manufacture of paper and rayon), bleaching cotton and textiles,
2. in the manufacture of dyes, drugs and organic compounds such as CCl4, CHCl3, DDT, refrigerants, etc.
3. in the extraction of gold and platinum.
4. in sterilising drinking water and
5. preparation of poisonous gases such as phosgene (COCl2), tear gas (CCl3NO2), mustard gas (ClCH2CH2SCH2CH2Cl).
3. Hydrogen chloride
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
HYDROGEN CHLORIDE (HCl)
Preparation :
By heating a halide with concentrated acid (Laboratory method) :
NaCl + H2SO4![]() NaHSO4 + HCl
NaHSO4 + HCl
NaHSO4 + NaCl ![]() Na2SO4 + HCl
Na2SO4 + HCl
This method is called as salt cake method as it involves the formation of NaHSO4 (salt cake).
HCl cannot be dried over P2O5 (P4O10) or quick lime since they react with gas chemically.
CaO + 2HCl ® CaCl2 + H2O
P4O10 + 3HCl ® POCl3 + 3HPO3
HCl is, hence dried by passing through concentrated H2SO4 .
Properties :
(i) It is a colourless, pungent smelling gases with acidic tastes.
(ii) It is easily liqueflied to a colourless liquid (b.p. 189 K) and freezes to a white crystalline solid (f.p. 159 K).
(iii) It is quite soluble in water.
HCl ionises as below : HCl(g) + H2O (l) ® H3O+ (aq) + Cl– (aq) ; Ka = 107
It aqueous solution is called hydrochloric acid. High value of dissociation constant (Ka) indicates that it is a strong acid in water.
When three parts of concentrated HCl and one part of concentrated HNO3 are mixed, aqua regia is formed which is used for dissolving noble metals, e.g., gold, platinum.
Au + 4 H+ + NO3– + 4 Cl– ® [AuCl4]– + NO + 2 H2O
3 Pt + 16 H+ + 4 NO3– + 18 Cl– 3 [PtCl6]2– + 4 NO + 8 H2O
It reacts with ammonia forming white fumes of NH4Cl
NH3 + HCl ® NH4Cl
(iv) It decomposes salt of weaker acids.
Na2CO3 + 2HCl ® 2NaCl + H2O + CO2
NaHCO3 + HCl ® NaCl + H2O + CO2
Na2SO3 + 2HCl ® 2NaCl + H2O+ SO2
Uses :
1. HCl is used in preparation of Cl2, chlorides, aqua regia, glucose, (from corn starch),
2. It is used in medicines, laboratory as reagents, cleaning metal surfaces before soldering or electroplating.
3. It is used for extracting glue from bones and purifying bone black.
4. Oxoacids of halogens
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
OXY-ACIDS OF Halogens
Fluorine forms only one oxoacid, HOF due to high electronegativity and small size. Other halogens form a number of oxoacids which are stable only in aqueous solutions or in the form of their salts. They can not be isolated in pure form.
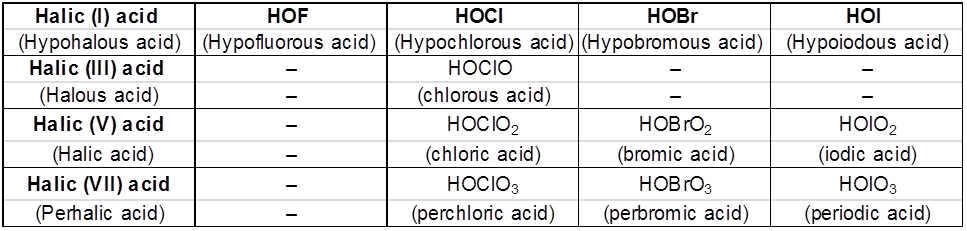
Some important order
(a) Acid strength
(i) HI > HBr > HCl > HF
(ii) HOCl > HOBr > HOI
(iii) HClO4 > HClO3 >HClO2 > HClO
(b) Oxidising powder
(i) F2 > Cl2 > Br2 > I2
(ii) BrO4– > IO4– > ClO4– (According to electrode potential)
(c) Order of disproportionations
3 XO– ® 2X– + XO3– (hypohalite ion) ;
IO– > BrO– > ClO–
5. Interhalogen compounds
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Interhalogen compounds
We know that halogen atoms have different electronegativity. Due to this difference in electronegativity the halogen atoms combine with each other and give rise to the formation of binary covalent compounds, which are called interhalogen compounds. These are of four types.
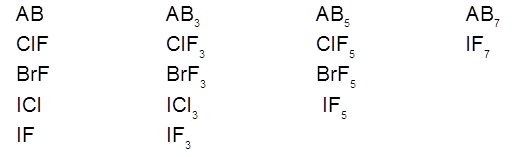
Preparation :
Cl2 + F2 (equal volumes) ![]() 2ClF
2ClF
Cl2 + 3F2 (excess) ![]() 2ClF3
2ClF3
I2 + Cl2 ® 2ICl ;
(equimolar)
Diluted with water :
Br2 (g) + 3F2 ® 2BrF3
F2 is diluted with N2 :
I2 + 3F2 ![]() 2IF3
2IF3
F2 is taken in freon :
Br2 + 5F2 (excess) ® 2BrF5
Properties :
(i) These compounds may be gases, liquids or solids.
Gases : ClF, BrF, ClF3 , IF7
Liquids : BrF3, BrF5
Solids : ICl, IBr, IF3, ICl3.
(ii) All interhalogens are covalent molecules and are diamagnetic in nature since all the valence electrons present as bonding or non-bonding electrons are paired.
(iii) The boiling points increases with the increase in the electronegativity difference between A and B atoms.
(iv) Interhalogen compounds are more reactive than the parent halogens but less reactive than F2.
(v) Hydrolysis : All these undergo hydrolysis giving halide ion derived from the smaller halogen and a hypohalite (when AB), halite (when AB3), halate (when AB5), and perhalate (when AB7) anion derived from the larger halogen.
AB + H2O ® HB + HOA
BrCl + H2O ® HCl + HOBr
ICl + H2O ® HCl + HIO2
Uses :
(i) These compounds can be used as non aqueous solvents.
(ii) Interhalogen compounds are very useful fluorinating agents.
(iii) ClF3 and BrF3 are used for the production of UF6 in the enrichment of 235U.
U(s) + 3 ClF3 (l) ® UF6 (g) + 3 ClF (g)
6. Group 18 elements
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Group 18 Elements : The Zero Group Family
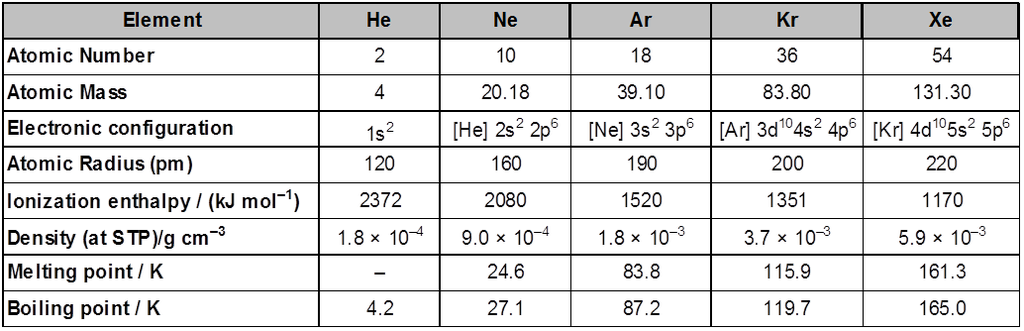
Group 18 consists of six elements: helium, neon, argon, krypton , xenon and radon . All these are gases and chemically unreactive. They form very few compounds . Because of this they are termed noble gases.
Occurrence : All the noble gases except radon occur in the atmosphere. Their atmospheric abundance in dry air is ~ 1% by volume of which argon is the major constituent. Helium and sometimes neon are found in minerals of radioactive origin e.g., pitchblende, monazite, cleveite. The main commercial source of helium is natural gas. Xenon and radon are the rarest elements of the group. Radon is obtained as a decay product of 226Ra.
![]()
Most abundant element in air is Ar. Order of abundance in the air is Ar > Ne > Kr > He > Xe.
Electronic Configuration : All noble gases have general electronic configuration ns2np6 except helium which has 1s2 . Many of the properties of noble gases including their inactive nature are ascribed to their closed shell structures.
Ionisation Enthalpy : Due to stable electronic configuration these gases exhibit very high ionisation enthalpy . However, it decreases down the group with increases in atomic size.
Atomic Radii : Atomic radii increase down the group with increase in atomic number.
Electron Gain Enthalpy : Since noble gases have stable electronic configurations, they have no tendency to accept the electron and therefore, have larger positive values of electron gain enthalpy.
Physical properties : All the noble gases are mono-atomic. They are colourless, and tasteless. They are sparingly soluble in water. They have very low melting and boiling points because the only type of interatomic interaction in these elements is weak dispersion forces,. Helium has the lowest boiling point (4.2K) of any known substance. It has a unusual property of diffusing through most commonly used laboratory materials such as rubber, glass or plastics.
Atomic & physical properties
Chemical Properties :
In general, noble gases are least reactive. Their inertness to chemical reactivity is attributed to the following reasons :
(i) The noble gases except helium (1s2) have completely filled ns2 np6 electronic configuration in their valence shell.
(ii) They have high ionisation enthalpy and more positive electron gain enthalpy.
The reactivity of noble gases has been investigated occasionally ever since their discovery, but all attempt to force them to react to form the compounds were unsuccessful for quite a few years. In March 1962, Neil Bartlett, then at the University of British Columbia, observed the reaction of a noble gas. First , he prepared a red compound which is formulated as O2+ PtF6–. He , then realised that the first ionisation enthalpy of molecular oxygen (1175 kJ mol –1) was almost identical with that xenon (1170 kJ mol–1). He made efforts to prepare same type of compound with Xe+ PtF6– by mixing Pt F6 and Xenon. After this discovery, a number of xenon compounds mainly with most electronegative elements like fluorine and oxygen, have been synthesised.
The compounds of krypton are fewer. Only the difluoride (KrF2) has been studied in detail. Compounds of radon have not been isolated but only identified (e.g., RnF2) by radiotracer technique. No true compounds of Ar, Ne or He are yet known .
7. Compounds of Xenon
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
COMPOUNDS OF XENON
Xenon–FLUORINE compounds :
All fluorides of xenon are white solids. They are volatile, readily subliming at room temperature (298 K)
![]()
They can be stored indefinitely in nickel or monel metal containers.
Xenon difluoride
Xe + F2 (2 : 1 mixture) ![]() XeF2 .
XeF2 .
It is soluble in water, giving solution 0.15 M at 0ºC. The solution which have pungent smell due to XeF2 are powerful oxidizing agents.
XeF2(aq) + 2H+ + 2e– ® Xe + 2HF(aq) ; Eº = + 2.64 V
XeF2 + H2 ®2 HF + Xe
Hydrolysis is slow in water due to dissolution of XeF2 in HF formed, but is rapid in basic solution.
2Xe F2 + 2H2O ®2 Xe + 4HF + O2
XeF2 + 2OH– ® Xe + ![]() O2 + 2F– + H2O
O2 + 2F– + H2O
It acts as fluoride ion donor XeF2 (Lewis base) + PF5 (Lewis acid) [XeF]+ [PF6]–
Xenon tetrafluoride
Xe + 2F2 (1 : 5 mixture) 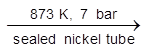 XeF4
XeF4
XeF4 reacts violently with water, giving dangerously explosive XeO3.
6XeF4 + 12H2O ® 4Xe + 2 XeO3 + 24 HF + 3O2
XeF4 can act as F– acceptor as well as F– donors and thus form anionic species in reactions such as :
XeF4 + NaF ® Na+ XeF5–
XeF4 + SbF5 ® [XeF3]+ [SbF6]–
Xenon hexafluoride
Xe + 3F2 (1 : 20 mixture) ![]() XeF6
XeF6
XeF4 + O2 F2 ® XeF6 + O2
XeF6 reacts violently with water but slow hydrolysis with atmospheric moisture giving XeO3.
XeF6 + 6 H2O ® XeO3 + 6HF (complete hydrolysis)
With small quantities of water, partial hydrolysis takes place.
XeF6 + H2O ® XeOF4 (colourless liquid) + 2HF
XeF6 + 2 H2O ® XeO2F2 + 4HF
XeF6 reacts with fluoride ion donors to form fluoroanions.
XeF6 + MF® M+ [XeF7]– (M = Na, K, Rb or Cs)
Xenon–oxygen compounds :
Hydrolysis of XeF4 and XeF6 with water gives XeO3.
6 XeF4 + 12 H2O ® 4 Xe + 2 XeO3 + 24 HF + 3 O2
XeF6 + 3 H2O ® XeO3 + 6 HF
Partial hydrolysis of XeF6 gives oxyfluorides, XeOF4 and XeO2F2 .
XeF6 + H2O ®XeOF4 + 2 HF
XeF6 + 2 H2O ® XeO2F2 + 4 HF
XeO3 is a colourless explosive solid and has a pyramidal molecular structure. XeOF4 is a colourless volatile liquid and has a square pyramidal molecular structure.
Uses :
Helium is a non–inflammable and light gas. Hence, it is used in filling balloons for meteorological observations. It is also used in gas–cooled nuclear reactors. Liquid helium (b.p.4.2 K) finds use as cryogenic agent for carrying out various experiments at low temperatures. It is used to produce and sustain powerful superconducting magnets which form an essential part of modern NMR spectrometers and Magnetic Resonance Imaging (MRI) systems for clinical diagnosis. It is used as a diluent for oxygen in modern diving apparatus because of its very low solubility in blood.
Neon is used in discharge tubes and fluorescent bulbs for advertisement display purposes. Neon bulbs are used in botanical gardens and in green houses.
Argon is used mainly to provide an inert atmosphere in high temperature metallurgical processes (arc welding of metals or alloys) and for filling electric bulbs. It is also used in the laboratory for handlsing substances that are air–sensitive.
Xenon and Krypton are used in light bulbs designed for special purposes

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
