- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Ammonia
Preparation :
(i) Ammonia is present in small quantities in air and soil where it is formed by the decay of nitrogenous orgainc matter e.g., urea.
NH2CONH2 + 2 H2O —® (NH4)2CO3 ![]() 2 NH3 + H2O + CO2
2 NH3 + H2O + CO2
(ii) On a small scale ammonia is obtaned from ammonia salts which decompose when treated with caustic soda or lime.
2 NH4Cl + Ca (OH)2 —® 2 NH3 + 2 H2O + CaCl2
(NH4)2 SO4 + 2 NaOH —® 2 NH3 + 2 H2O + Na2SO4
(iii) On a large scale, ammonia is manufactured by Haber’s process.
N2 (g) + 3 H2 (g) —® 2 NH3 (g) ; Df H– = – 46.1 kJ mol–1
In accordance with Le Chatelier’s principle, high pressure would favour the formation of ammonia. The optimum conditions for the production of ammonia are a pressure of 200 × 105 Pa (about 200 atm) , a temperature of ~ 700 K and the use of a catalyst such as iron oxide with small amounts of K2O and Al2O3 to increase the rate of attainment of equilibrium.
(iv) By hydrolysis of metal nitrides like AIN or Mg3N2
AIN + NaOH + H2O —® NaAIO2 + NH3
For drying, dehydrating agents like H2SO4 , P2O5 or CaCl2 can not be used as these react with NH3.
2NH3 + H2SO4 —® (NH4)2SO4 ; 6NH3 + P2O5 + 3H2O —® 2(NH4)3PO4
CaCl2 + 8NH3 —® CaCl2 8NH3 (Adduct)
So quicklime (CaO) is used for drying of NH3 .
CaO + H2O —® Ca(OH)2
Properties :
Physical properties : Ammonia is a colourless gas with a pungent odour. Its freezing and boiling points are 198.4 and 239.7 K respectively. In the solid and liquid states , it is associated through hydrogen bonds and that accounts for its higher melting and boiling points than expected on the basis of its molecular mass. The ammonia molecule is trigonal pyramidal with the nitrogen atom at the apex. It has three bond pairs and one lone pair of electrons as shown in the structure.
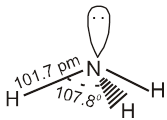
Ammonia gas is highly soluble in water. Its aqueous solution is weakly basic due to the formation of OH– ions. NH3 (g) + H2O (1) ![]() NH4+ (aq) + OH– (aq)
NH4+ (aq) + OH– (aq)
Chemical properties :
(i) It forms ammonium salts with acids , e.g., NH4Cl , (NH4)2 SO4 , etc. As a weak base , it precipitates the hydroxides of many metals from their salt solutions. For example ,
2 FeCl3 (aq) + 3 NH4OH (aq) —® Fe2O3 . xH2O (s) + 3 NH4Cl (aq)
(brown ppt)
(ii) The presence of lone pair of electrons on the nitrogen atoms of the ammonia molecule makes it a Lewis base. It donates the electrons pair and forms linkage with metal ions forming complex compounds.
Cu2+ (aq) + 4 NH3 (aq) ![]() [Cu(NH3)4]2+ (aq)
[Cu(NH3)4]2+ (aq)
(blue) (deep blue)
Ag+ (aq) + Cl–1 (aq) ![]() AgCl (s)
AgCl (s)
(colourless) (white ppt)
AgCl (s) + 2 NH3 (aq) —® [Ag (NH3)2]Cl (aq)
(white ppt) (colourless)
Test of ammonia/ammonium salts :
When NH3 gas is passed into the colourless solution of Nessler’s reagent a brown precipitate or coloration is formed. This is a test for NH3 gas.
2K2HgI4 + 3KOH + NH3 —® H2N·HgO·HgI ↓(brown) + 7KI + 2H2O
Uses :
1. Liquid ammonia is used as a refrigerant.
2. For the production of ammonium fertilizers such as ammonium sulphate, ammonium phosphate, ammonium nitrate, urea etc.
3. For removing grease because NH4OH dissolves grease.
4. For manufacture of HNO3 by the Ostwald process.
5. As a laboratory reagent.
6. In the production of artificial rayon, silk, nylon etc

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
