- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Classification of crystalline solids
STUDY OF CRYSTALS :
Crystal : A crystal is a homogeneous portion of a solid substance made by regular pattern of structural units bonded by plane surface making definite angles with each other.
Space lattice : The arrangement of constituents like atom, ions and molecules in different sites in three dimensional space is called space lattice.
Unit cell : The smallest repeating unit in space lattice which when repeats over and over again, results in a crystal of the given substance called unit cell.
Properties of a cube :
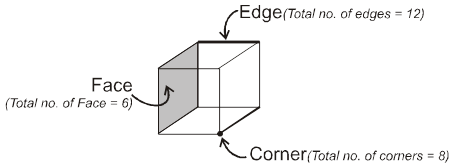
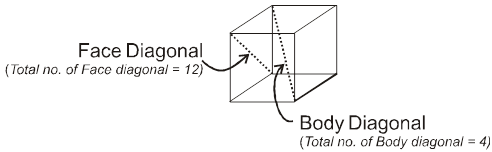
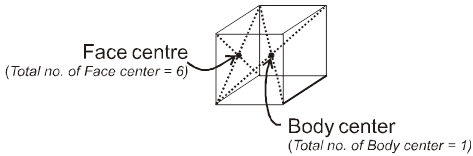
Face : The plane surface of the crystal are called faces.
Edge : An edge is formed by the intersection of two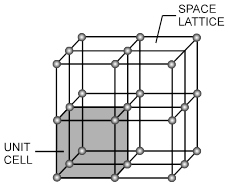 adjacent faces.
adjacent faces.
Interfacial angles : The angle between the perpendiculars two intersecting faces called interfacial angles.
Unit Cell in two dimensions :
![]()
Now in order to uniquely explain a regular arrangement in two dimensions we need the help of three parameters, two distance parameters and one angular parameter. Based upon their different relationships we can define different cases
Case ‘A’ (a = b) angle = 90º

The unit cell in such a case is a square. such square side by side we will obtain the entire two dimensional arrangement.
Case ‘B’(a ¹ b) angle = 90º

Placing The unit cell formed in this case is a rectangle.
Unit cell in three dimensions :
It has six parameters, 3-distance parameters and 3-angular parameter.
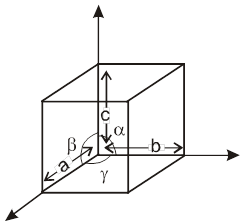
a, b, c are lengths of unit cell (also known as the crystallographic axes). a, b, g are known as the crystallographic angles.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
