- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Solubility of gases in liquids
Factors Affecting Solubility of Gas In Liquid :
(i) Nature of gas
(ii) Nature of liquid
(iii) Temperature
(iv) Pressure
Henry's Law (effect of pressure on solubility of gases in liquids) :
Statement : The solubility of a gas in a liquid at a given temperature is directly proportional to its partial pressure at which it is dissolved.
Let x = Mole fraction of unreacted gas in solution at a given temperature as a measure of its solubility.
p = Partial pressure of gas in equilibrium with the solution.
By Henry's law: x µ p or p µ x
That is; p = KHx or x = ![]() ,
,
where KH = Henry's law constant.
Characteristics of Henry's Law constant (KH).
(i) Unit same as those of pressure: torr or bar.
(ii) Different gases have different value of KH for the same solvent.
(iii) The KH value of a gas is different in different solvents and it increase with the increase in temperature.
(iv) Higher the value of KH of a gas, lower will be its solubility. Since, x = ![]() .
.
Plot of p Vs x is a straight line passing through the origin with slope equal to KH .
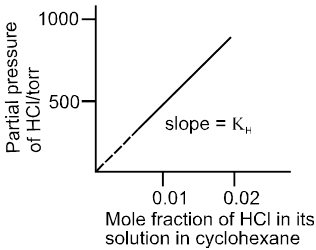
Plot of p Vs x for solution of HCl in cyclohexane.
Note : If a mixture of gases is brought in contact with solvent each constituent gas dissolves in proportion to its partial pressure. It means Henry's law applies to each gas independent of the pressure of other gas.
Effect of temperature : Solubility of gases in liquids decreases with rise in temperature.
Explanation : When dissolved, the gas molecules are present in liquid phase and the process of dissolution can be considered similar to condensation and heat is evolved in this process. We have learnt that dissolution process involves dynamic equilibrium and thus must follow Le Chatelier's principle. As dissolution of gases in liquids is an exothermic process, the solubility should decrease with increase of temperature.
Note : KH values for both N2 and O2 increase with increase of temperature indicating that the solubility of gases increases with decrease of temperature. It is due to this reason that aquatic spcies are more comfortable in cold water rather than warm water.
Applications of Henry's law : It has several applications in biological and industrial phenomena.
(i) To increase the solubility of CO2 in soft drinks and soda water the bottle is sealed under high pressure.
(ii) Scuba divers must cope with high concentrations of dissolved gases while breathing air at high pressure underwater. Increased pressure increases the solubility of atmosphere gases in blood. When the divers come towards surface, the pressure is gradually decreased. This releases the dissolved gases and leads to the formation of bubbles of nitrogen in the blood. This blocks capillaries and creates a medical condition known as bends, which are painful and dangerous to life. To avoid bends, as well as, the toxic effects of high concentrations of nitrogen in the blood, the tanks used by scuba divers are filled with air diluted with helium (11.7% helium, 56.2% nitrogen and 32.1% oxygen).
(iii) At high altitudes the partial pressure of oxygen is less than that at the ground level. This leads to low concentrations of oxygen in the blood and tissues of people living at high altitudes or climbers. Low blood oxygen causes climbers to become weak and unable to think clearly, symptoms of a condition known as anoxia.
Summary

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
