Cell cycle
Chapter 10
Cell Cycle and cell division
Cell Cycle
Cells, and all living things for that matter, exhibit growth and reproduction. Each parental cell produces two daughter cells every time it divides, which is how all cells reproduce. A new cell population can be created by the growth and division of a single parental cell and its offspring, which is accomplished by the newly generated daughter cells. In other words, repeated cycles of growth and division enable the formation of structures made up of millions of cells from a single cell.
All living things go through the process of cell division. DNA replication and cell proliferation also happen when a cell divides. To ensure proper division and the production of offspring cells with complete genomes, processes like cell division, DNA replication, and cell development are coordinated. Cell cycle refers to the series of actions that a cell takes to reproduce its genome, synthesise the other components of the cell, and ultimately divide into two daughter cells. DNA synthesis only takes place during one particular stage of the cell cycle, despite the fact that cell growth (as measured by cytoplasmic expansion) is a constant process.During cell division, a complicated chain of processes transfers the replicated chromosomes (DNA) to the daughter nuclei. These occurrences are genetically determined.
In order to form two genetically identical cells, cells go through a series of carefully timed and regulated steps of growth, DNA replication, and division. Interphase and the mitotic phase are the two main stages of the cell cycle. The cell develops and DNA replication occurs during interphase. The cell divides and the replicated DNA and cytoplasm are separated during the mitotic phase.
The replication and reproduction of cells, whether in eukaryotes or prokaryotes, happens during the cell cycle. Although it serves several purposes for organisms, it ultimately ensures their survival. Prokaryotes can continue to exist by dividing into two new daughter cells thanks to a process termed binary fission in the cell cycle.
Reproduction, growth, and gamete creation are the three primary purposes of cell division. For asexual reproduction, growth, repair, and regeneration, mitosis is necessary. The bodies must create new cells—and permit the death of old cells—in order to expand and develop. The process of healing an injury also requires cell division.If cells were unable to divide and produce new cells, living organisms would never be able to regenerate skin cells to treat rashes or regrow a fingernail.
What is a cell ?
Chapter 8: Cell the unit of life
1. What is a Cell?
All living organisms are composed of cells. Cells are the building blocks of any living body. It is the basic structural as well as the functional unit of life. Some living organisms are made up of only a single cell and are thus known as unicellular organisms (e.g. bacteria), while many other organisms are composed of numerous cells and are therefore called multicellular organisms (e.g. plants and animals).
The invention of the microscope in the 17th century paved the way for the discovery of cells. Thecell was firstdiscovered by Dutch scientist Antonie van Leeuwenhoek. He was the first person to observe and describe a live bacterial cell. He is also known as the father of microbiology. Later, the discovery of the nucleus in a cell, by Robert Brown was another significant milestone in the field of microbiology.
Cell Theory
Cell Theory
In the year 1838, a German botanist called Matthias Schleiden discovered that all plant tissues are made up of different types of cells. A British Zoologist, Theodore Schwann, who was a contemporary of Schleiden, reported the presence of athin outer layer in the animal cells which is now known as the plasma membrane. Schwann also discovered that along with plasma membrane,an additional unique outer layer is also found exclusively in plant cells i.e. the cell wall. Both Schleiden and Schwann together postulated the Cell Theory, based on their findings. However, this theory did not give any indication of the genesis of cells.
In 1855, a German biologist named Rudolf Virchow first explained the origin of cells from pre-existing cells. This led to the modification of the original cell theory proposed by Schleiden and Schwann which is today understood as:
- All living organisms are made up of cells and cell products.
- Every cell is made up of pre-existing cells.
An overview of cell
An overview of the cell
If we observe an onion peel or human cheek cells under a microscope, we will find that the cells in an onion peel show a distinct cell wall as its outermost boundary. The cell membrane lies just inwards to the cell wall. On the other hand, in a human cheek cell, the outermost delimiting structure is the cell membrane. In both, plant and animal cells, a centrally located dense membrane-bound structure called the nucleus is present. This nucleus is known to contain hereditary material i.e. DNA.
Cells that contain a membrane-bound nucleus are known as Eukaryotic cells, whereas the cells in which the nucleus is not defined by a membrane are called Prokaryotic cells. In both prokaryotic as well as eukaryotic cells, the volume of the cell is composed of a semi-fluid matrix called the Cytoplasm. This cytoplasm is the primary site of cellular activities which are important for a living cell.
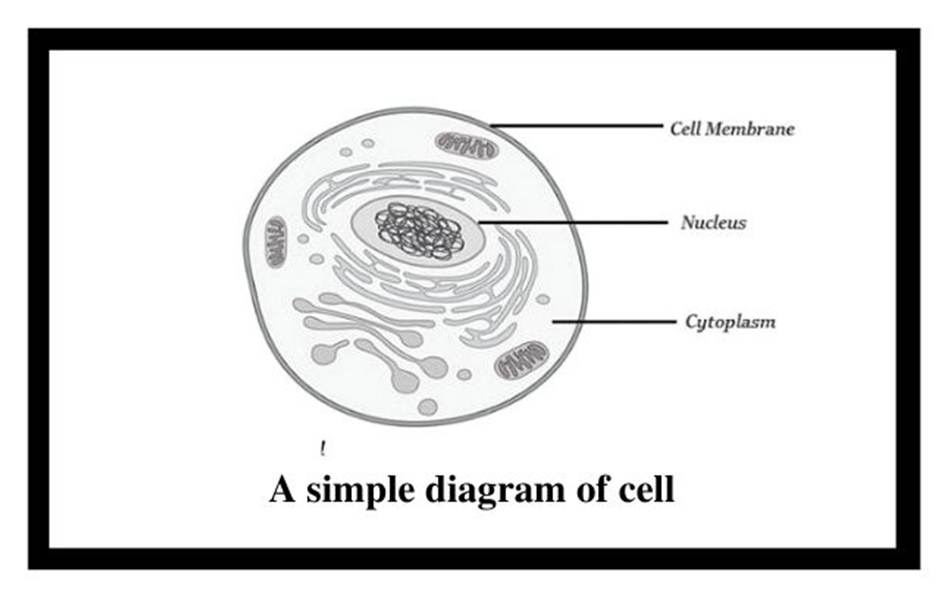
Figure 1: Basic structure of a cell.
Along with the nucleus, a eukaryotic cell also contains cell organelles which are distinct membrane-bound structures. These organelles are responsible for carrying out different metabolic functions. These cell organelles are absent in prokaryotic cells. Some important organelles of a cell are:
a) Mitochondria: are responsible for generating the majority of the chemical energy required by the cell's metabolic activities.
b) Endoplasmic Reticulum: is responsible for protein synthesis.
c) Golgi complex: aids in the processing and packaging of proteins and lipid molecules, particularly proteins destined for cell export.
d) Lysosomes: act as the digestive system of a cell.
e) Vacuoles: help in the storage and disposal of various waste substances in a cell.
f) Ribosomes: are the site for protein synthesis in the cell. They are not bound by a membrane.
g) Centrosome: is also not bound by a membrane. It is present in animal cells and helps in cell division.
Cells can be differentiated based on size, shape, and activities. The smallest cell i.e. Mycoplasma is only 0.3µm long and is even smaller than bacteria. On the other hand, an Ostrich egg, the largest known single cell, is about 15cm to 18 cm long and wide. Cells can be of varying shapes such asdisc-shaped (RBCs), columnar (Goblet cells in the intestine), polygonal (Hydra), cuboid (kidneys), branched (Neuron), elongated (tracheid),and even irregular (Amoeba and WBCs).
Prokaryotic Cells
Prokaryotic cells
The best-known examples of prokaryotic cells are bacteria, blue-green algae, PPLO, and Mycoplasma. Prokaryotic cells multiply more rapidly than eukaryotic cells and are also smaller in size. Their basic shapes are rod-like (bacillus), comma-shaped (vibrio), spherical (coccus), and spiral (spirillum). Despite exhibiting great diversity in shapes, their basic organization remains similar. Except for Mycoplasma, all prokaryotes have a cell wall surrounding their cell membrane. The cytoplasm contains a non-membrane-bound nucleus, containing naked genetic material. Apart from genomic DNA additional circular DNA is also present in prokaryotes known as Plasmid DNA. This plasmid DNA is responsible for imparting unique phenotypes to bacteria. The plasmid DNA also offers antibiotic resistance to the bacterium, which is helpful in case of bacterial transformation. Except for Ribosomes, no other organelle is seen in prokaryotes.
A prokaryotic cell envelope is composed of a tightly bound triple-layered structure. The outermost part of this triple layer is the glycocalyx followed by a cell wall and then the plasma membrane. Each layer has a specific function. Based on the distinctions in the cell envelopes, bacteria are classified into 2 groups namely Gram-positive and Gram-negative. Due to the differences in cell envelops, the bacteria which respond to the Gram stain are regarded as positive whereas the ones that do not take up the stain, are known as Gram-negative.
The composition of glycocalyx differs among different bacterial groups. In some groups, it appears as a loose slimy layer while in others it is present as a thick and sturdy capsule. The cell wall maintains the shape of the cell and provides resistance to the bacterium against disintegration or bursting. The plasma membrane establishes the connection of cells with the outside world. It is selectively permeable in both prokaryotes and eukaryotes.
A unique characteristic of Prokaryotes is the presence of Mesosomes, which are the infoldings of the plasma membrane into the cell. These infoldings can be tubular, lamellar, or vesicular in appearance. They are responsible for cell wall formation, DNA replication, respiration, and secretion processes. In cyanobacteria, other cell membrane modifications are seen which extend into the cytoplasm. They contain pigments and are known as Chromatophores.
Motile bacterial cells possess thin filamentous extensions arising from the cell wall, known as Flagella. A single flagellum consists of a filament, a hook, and a basal body. Apart from flagella, Pili and Fimbriae are also found on the bacterial surface although they have no role to play in motility. Pili are elongated structures made up of proteins and Fimbriae are bristle-like structures erupting from the cell surface. They are known to help the bacteria attach to host tissues or surfaces.
The prokaryotic plasma membrane encloses ribosomes. These ribosomes consist of 2 subunits namely 50s (15nm) and 30s (20nm). Together they form the 70s ribosomal subunit. Ribosomes are known as the site of protein synthesis. They often attach themselves to a single mRNA and form a chain called polyribosome which eventually translates mRNA into proteins.
Prokaryotic cells store their unused or reserved material in specialized non-membranous structures called inclusion bodies which are present in the cytoplasm. Examples of inclusion bodies include glycogen granules, phosphate granules, and cyanophycean granules. In blue-green and purple photosynthetic bacteria, gas vacuoles are also present.
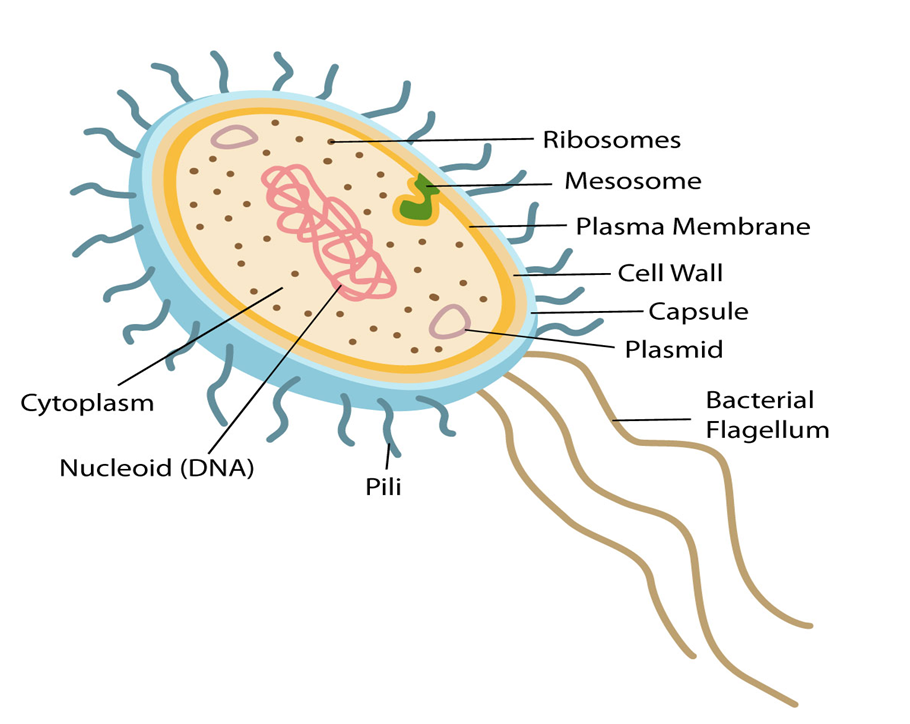
Figure 2: Features of a Prokaryotic cell.
Eukaryotic Cells
Eukaryotic Cells
Eukaryotes refer to any cell or organism having an identifiable nucleus. The nucleus of a eukaryotic cell is surrounded by a nuclear membrane, which contains well-defined chromosomes. Plants, animals, protists, and fungi are examples of eukaryotic cells. Chromosomes contain their genetic material. Eukaryotic cells have organelles such as the Golgi apparatus, Mitochondria, Ribosomes, and Nucleus. They have intricate locomotory and cytoskeletal features, as well.
Among eukaryotes, plant and animal cells show structural dissimilarities. Plant cells possess a cell wall, plastids, and a large central vacuole. An animal cell, on the other hand, possesses a centriole that is absent in the plant cell.
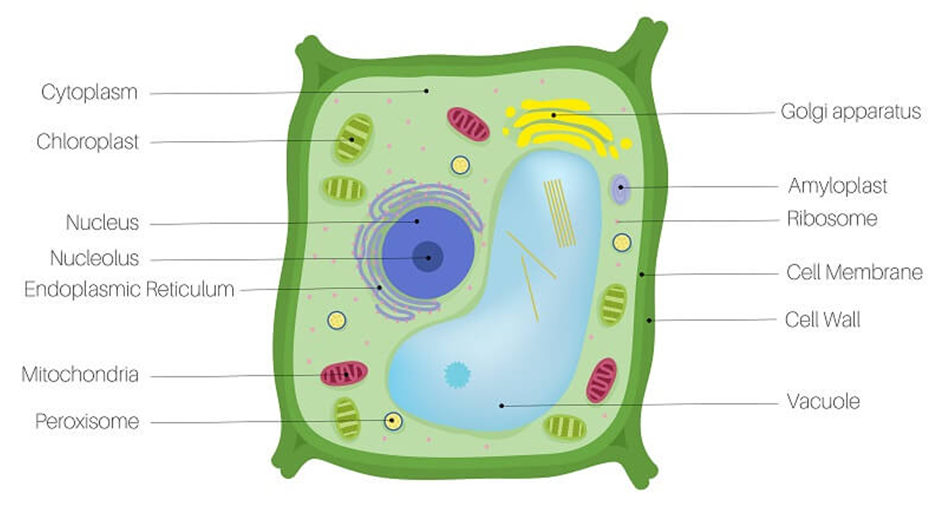
Figure 3: A plant cell.
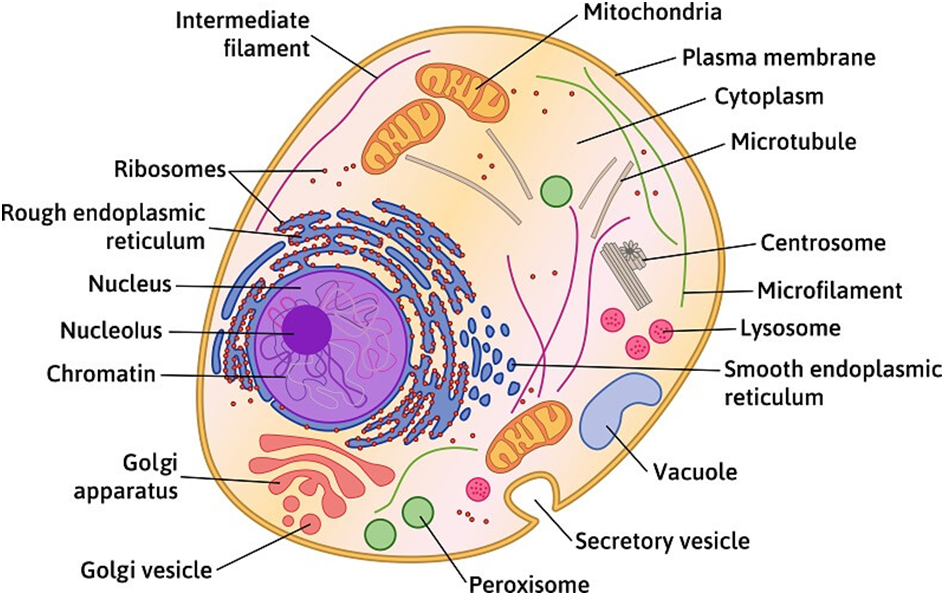
Figure 4: An Animal Cell.
(A)Cell Membrane:
The cell membrane, also known as the plasma membrane, separates the interior of the cell from the outside environment and is found in all cells. A semipermeable lipid bilayer makes up the cell membrane. The transfer of materials into and out of the cell is controlled by the cell membrane. In the late 1600s, Robert Hooke discovered the cell membrane.
Lipids and proteins make up the majority of the cell membrane. Phospholipids, which are organized in a bilayer, are the most important lipids. The lipids are also organized within the membrane with the polar heads on the outside and the hydrophobic tails on the inside, with the polar heads on the outside and the hydrophobic tails on the inside. This guarantees that saturated hydrocarbons' nonpolar tails are protected from the aqueous environment. Membranes include cholesterol in addition to phospholipids.
Biochemical analysis later revealed that cell membranes contain protein and carbohydrates as well. In different cell types, the protein-to-lipid ratio varies greatly. For example, the erythrocyte membrane contains around 52 percent protein and 40 percent lipids in humans.Membrane proteins are divided into two categories: integral and peripheral. Integral proteins are partially or completely buried in the membrane, whereas peripheral proteins are on the surface.
Singer and Nicolson (1972) provided an improved model of cell membrane construction that is now widely accepted as the fluid mosaic model. The quasi-fluid property of lipid, according to this, allows for lateral mobility of proteins within the total bilayer. The fluidity of a membrane is a measure of its capacity for internal movements. The fluid nature of the membrane is also critical for tasks such as cell proliferation, intercellular connection creation, secretion, endocytosis, and cell division.
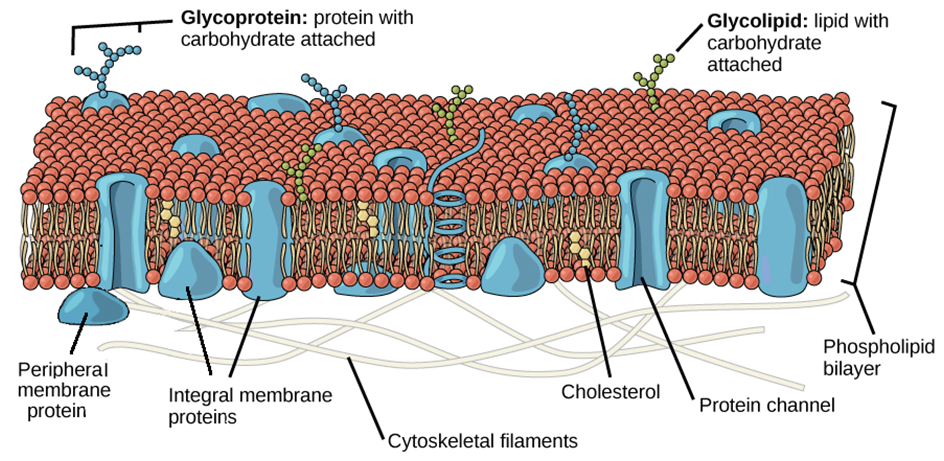
Figure 5: Fluid Mosaic Model of the plasma membrane.
The transport of molecules across the plasma membrane is one of the most significant tasks performed by this structure. Some molecules on each side of the membrane are selectively permeable through the membrane. The term "passive transport" refers to the ability of several molecules to travel across a membrane without requiring any energy. Simple diffusion along a concentration gradient, i.e. from a higher to a lower concentration, can transport neutral solutes across the membrane. Water can also travel from a greater to a lower concentration across this membrane. Osmosis is the movement of water by diffusion. As polar molecules cannot pass through the nonpolar lipid bilayer, they must be transported across the membrane by a membrane carrier protein.A few ions or molecules are transported across the membrane in the opposite direction of their concentration gradient, that is, from lower to higher concentration. Active transport, for example, is an energy-dependent activity that uses ATP as observed in a sodium-potassium pump.
(B) Cell Wall:
The cell wall is a non-living inflexible structure that is only found in plants and fungi. A cell wall not only gives the cell structure and protects it from mechanical damage and infection, but it also aids cell-to-cell communication and acts as a barrier to undesirable macromolecules. Algae have cellulose, galactans, mannans, and minerals like calcium carbonate in their cell walls, whereas other plants have cellulose, hemicellulose, pectins, and proteins. The primary wall of a young plant cell can develop, but as the cell grows, the primary wall shrinks, and the secondary wall forms on the inner (towards membrane) side of the cell.The middle lamella is a calcium pectate-based layer that binds or glues the surrounding cells together. Plasmodesmata, which connect the cytoplasm of neighboring cells, can pass through the cell wall and middle lamellae.
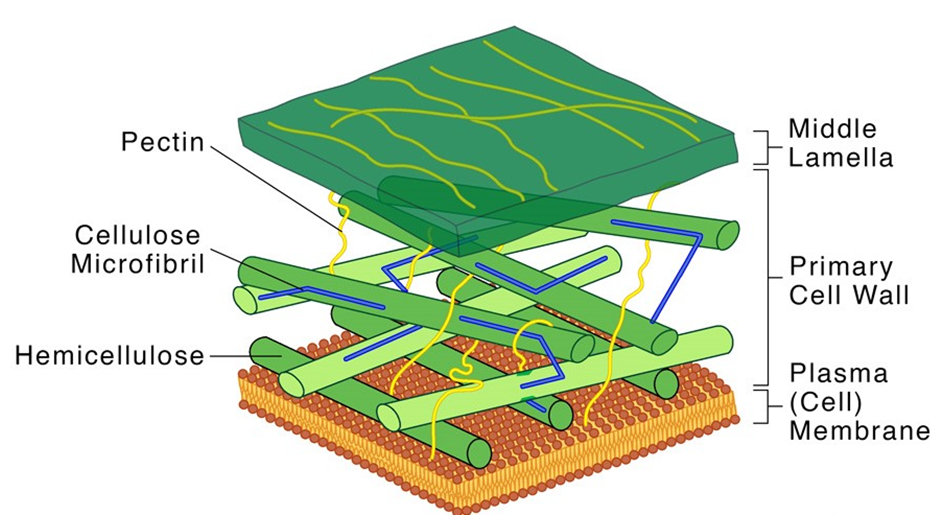
Figure 6: Structure of a Cell Wall.
Each membranous organelle has its structure and function, however, those organelles whose functions are coordinated, are grouped as an endomembrane system. Endoplasmic reticulum (ER), Golgi complex, lysosomes, and vacuoles are all part of the endomembrane system. The mitochondria, chloroplast, and peroxisomes are not considered part of the endomembrane system because their functions are not coordinated with those of the above organelles.
(C) Endoplasmic Reticulum (ER):
Upon observing a eukaryotic cell under an electron microscope, it was discovered that there is a network or reticulum of microscopic tubular structures dispersed throughout the cytoplasm.This network was identified as Endoplasmic Reticulum (ER). It divides the intracellular cavity into 2 parts namely, luminal (within ER) and extraluminal (cytoplasm). Ribosomes are frequently seen attached to the outer surface of ER. This is known as Rough ER (RER). RER is found in a lot of cells that are involved in protein synthesis and secretion. They are long and contiguous with the nucleus's outer membrane. Smooth ER (SER) refers to the absence of ribosomes on ER surface. The SER is the primary location for lipid synthesis. SER produces lipid-like steroidal hormones in animal cells.
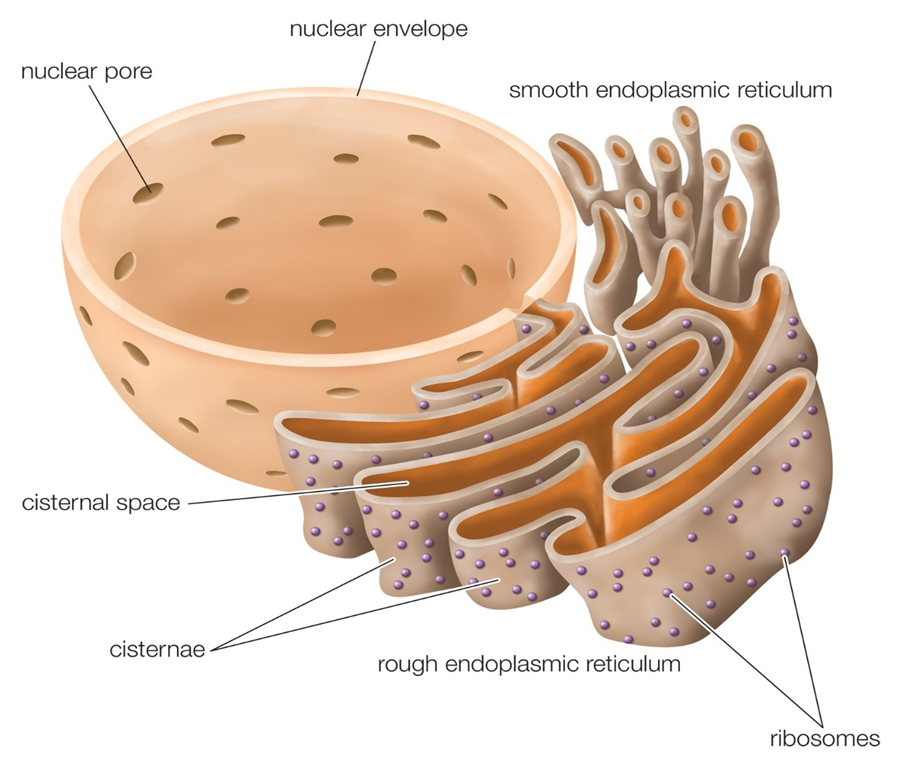
Figure 7: Endoplasmic Reticulum.
(D) Golgi apparatus:
In the year 1898, Camillo Golgi, an Italian researcher, first identified the heavily pigmented reticular structures near the nucleus. These entities were given the name Golgi bodies. They are made up of a large number of flat, disc-shaped sacs or cisternae with a diameter of 0.5m to 1.0m. These are piled one on top of the other. In a Golgi complex, there are a variety of cisternae. The Golgi cisternae are organized in a concentric pattern near the nucleus, with distinct convex Cis (forming face) and concave Trans (maturing face) face. The organelle's Cis and Trans faces are completely different, although they are linked. The Golgiapparatus is primarily responsible for packing materials for delivery to intracellular targets or secretion outside the cell.
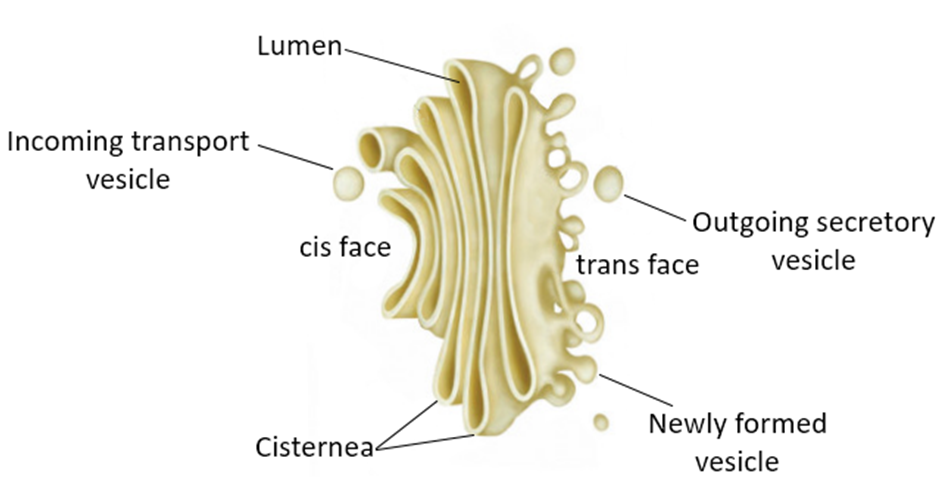
Figure 8: The Golgi apparatus.
(E) Lysosomes:
Lysosomes are membrane-bound vesicular organelles generated during the Golgi apparatus packaging process. Almost all types of hydrolytic enzymes (hydrolases – lipases, proteases, carbohydrases) were observed to be abundant in the isolated lysosomal vesicles, which are best active at acidic pH. Carbohydrates, proteins, lipids, and nucleic acids can all be digested by these enzymes. They are spheres made up of a lipid bilayer that encloses fluid containing a range of hydrolytic enzymes and have a simple structure.
Vacuoles:
In the cytoplasm, the vacuole is a membrane-bound compartment. It contains water, sap, excretory product, and other non-cellular elements. Tonoplast is a single membrane that separates the vacuole from the rest of the cell. Plant cells have vacuoles that can take up to 90% of the cell's volume. The tonoplast in plants accelerates the transport of ions and other materials over concentration gradients into the vacuole, resulting in a substantially higher concentration in the vacuole than in the cytoplasm. The contractile vacuole is crucial for osmoregulation and excretion in Amoeba. Food vacuoles are generated by engulfing food particles in various organisms, including protists.
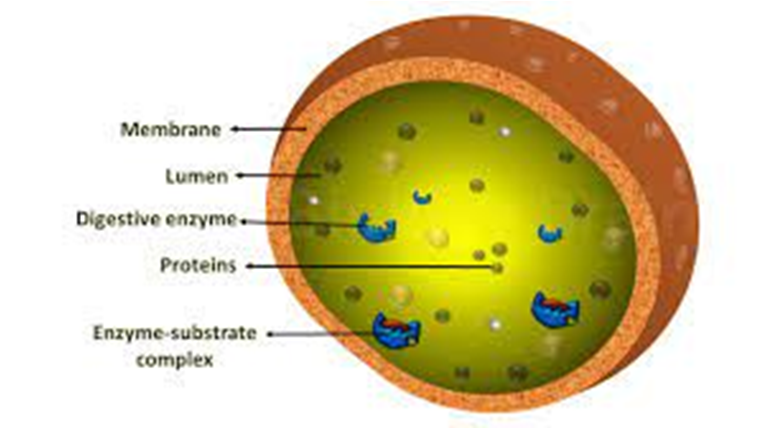
Figure 9: Lysosomes
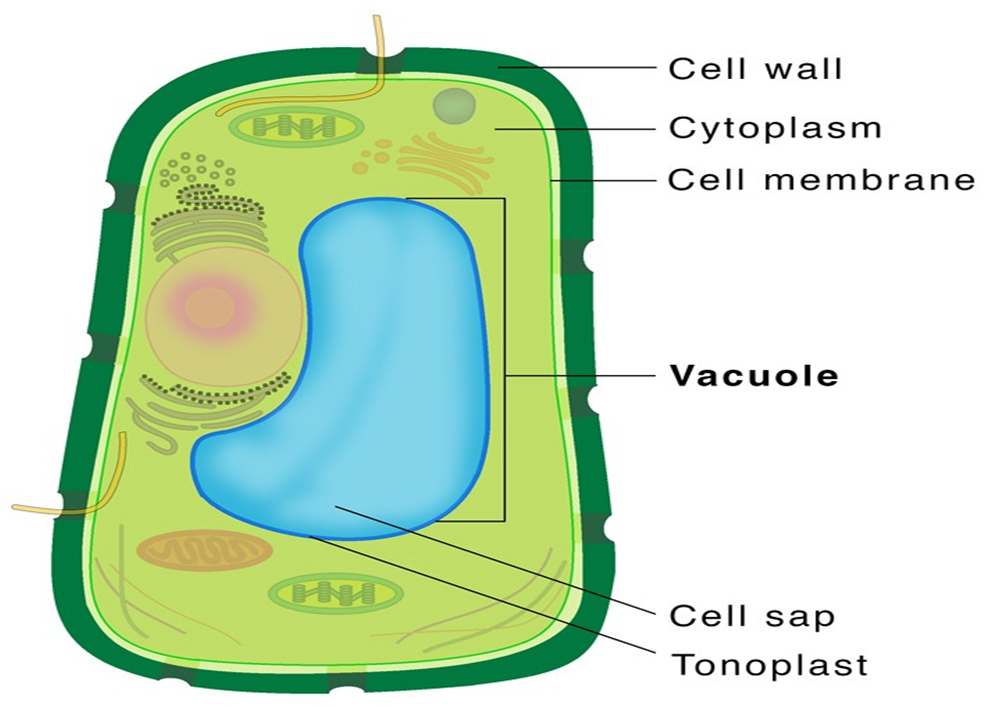
Figure 10: Vacuole.
(F) Mitochondria:
Mitochondria (plural: mitochondrion) are membrane-bound cell organelles that provide the majority of the chemical energy required to fuel the cell's metabolic activities. Adenosine triphosphate (ATP) is a tiny molecule that stores the chemical energy created by mitochondria. Each mitochondrion is a double membrane-bound structure, with the outer and inner membranes partitioning the lumen into two different aqueous compartments, the outer and inner compartments, respectively. The matrix is a solid, homogeneous substance that fills the inner compartment. The organelle's continuous limiting boundary is formed by the outer membrane. Towards the matrix, the inner membrane creates a series of infoldings called cristae (sing.: crista). The surface area is increased by the cristae. The enzymes linked with the two membranes are different.The two membranes each contain their own set of mitochondrial function-related enzymes. Aerobic respiration takes place in mitochondria. They are known as the 'power houses' of the cell because they produce cellular energy in the form of ATP. A single circular DNA molecule, a few RNA molecules, ribosomes (the 70S), and the components required for protein synthesis are also present in the mitochondrial matrix. Fission is the process through which mitochondria divide.
Mitochondria are difficult to see under a microscope unless they are stained specifically. The quantity of mitochondria per cell varies according to the cells' physiological activity. There is a great deal of variation in terms of shape and size as well.
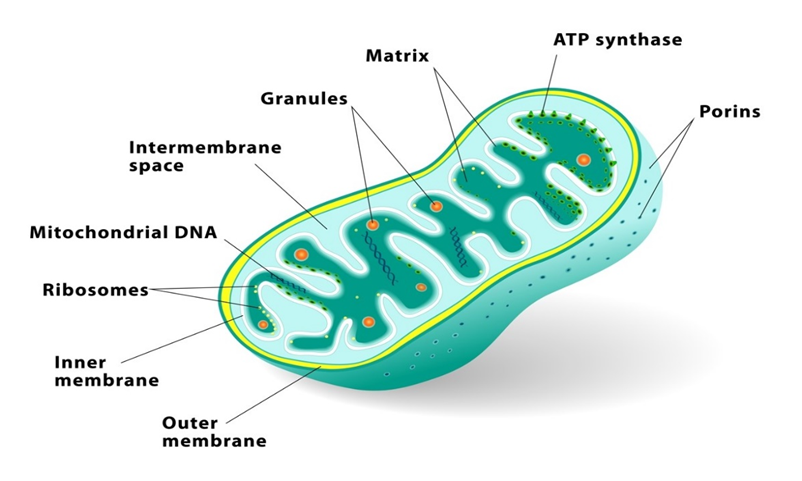
Figure 11: Mitochondria.
(G) Plastids:
All plant cells and euglenoids contain plasmids. Because they are big, they are easily visible under a microscope. They contain unique pigments, which give the plants their colors Plastids are divided into three types based on the pigments they contain: chloroplasts, chromoplasts, and leucoplasts. Chlorophyll and carotenoid pigments are found in chloroplasts, and they are important for capturing the light energy required for photosynthesis. Carotene, xanthophylls, and other fat-soluble carotenoid pigments are found in the chromoplasts. The plant's portion turns yellow, orange, or red as a result of this. Amyloplasts store carbohydrates (starch) inpotatoes, whereas elaioplasts store oils and lipids, and aleuroplasts store proteins.
The mesophyll cells of the leaves contain the majority of the chloroplasts in green plants. These organelles can be lens-shaped, oval, spherical, discoid, or even ribbon-like, with varying lengths (5-10m) and widths (2-4m). Their numbers range from one per cell in the green alga Chlamydomonas to 20-40 per cell in the mesophyll.
The chloroplasts, like mitochondria, a membrane-boundary bound. The inner chloroplast membrane is the less permeable of the two. The stroma is the compartment enclosed by the chloroplast's inner membrane. The stroma contains several thylakoids, which are flattened membrane sacs that are structured. Thylakoids are placed in stacks similar to grana (singular: granum) or intergranal thylakoids, which are coin piles. In addition, the stroma lamellae are flat membranous tubules that connect the thylakoids of the various grana. Thylakoids have a lumen that is enclosed by their membrane. The thylakoids contain chlorophyll pigments.Enzymes necessary for glucose and protein synthesis are found in the stroma of chloroplasts. It also contains ribosomes and tiny double-stranded circular DNA molecules.The chloroplast ribosomes (the 70S) are smaller than the cytoplasmic ribosomes (80S).
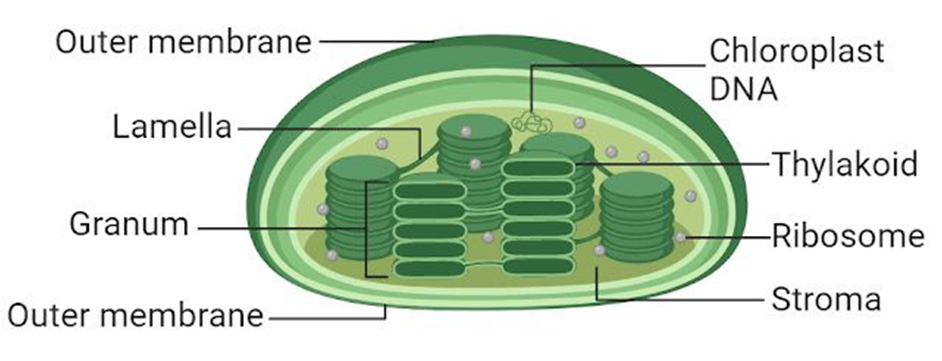
Figure 12: Plastids
(H) Ribosomes:
Ribosomes are granular structures that were first discovered as dense particles by George Palade (1953) through electron microscopy. They are made up of ribonucleic acid (RNA) and proteins and do not have a membrane surrounding them. The ribosomes of eukaryotes are, while those of prokaryotes are the 70S. There are two subunits in each ribosome: bigger and smaller subunits (Fig 8.9). The 60S and 40S are the two subunits of 80S ribosomes, while 50S and 30S are the subunits of 70S ribosomes.
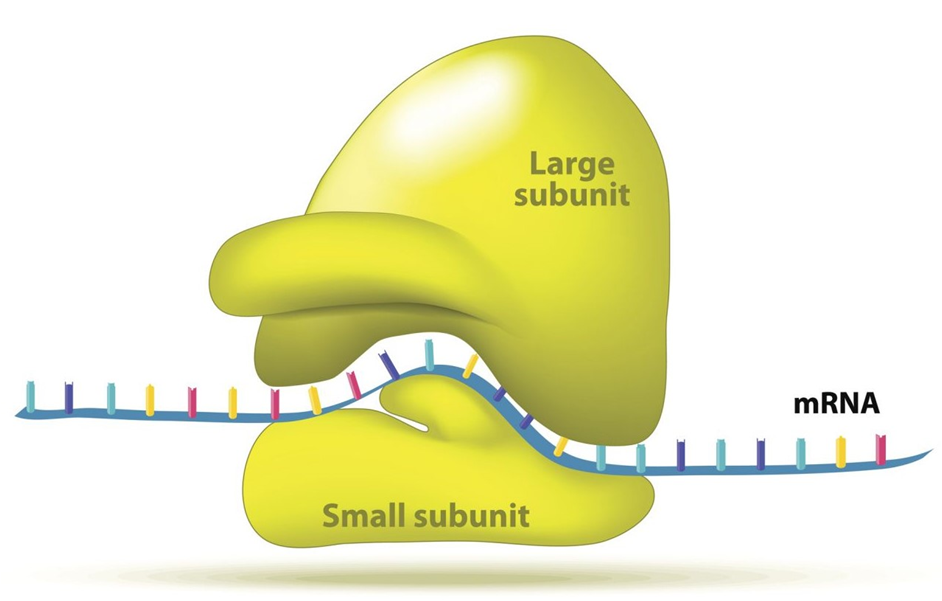
Figure 13: A Ribosome.
(I) Cytoskeleton:
The cytoskeleton is an intricate network of filamentous proteinaceous structures found in the cytoplasm that includes microtubules, microfilaments, and intermediate filaments. The cytoskeleton in a cell is engaged in a variety of tasks, including mechanical support, motility, and cell shape maintenance.
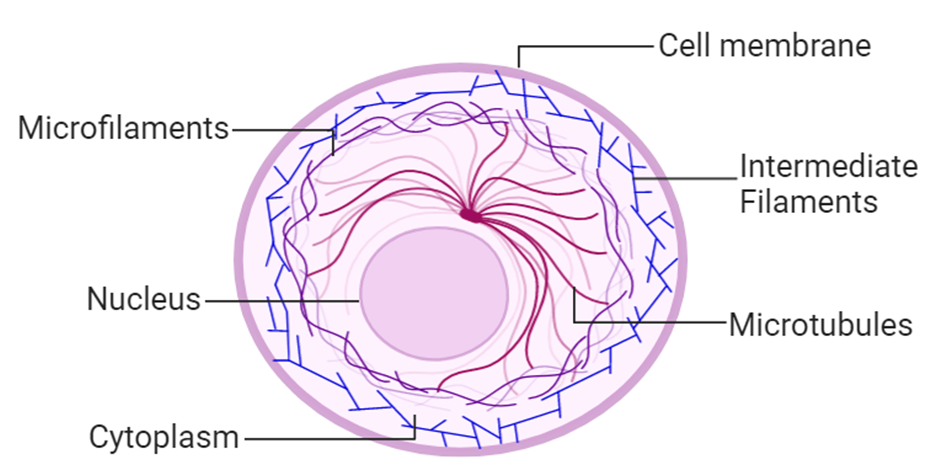
Figure 14: Cytoskeleton
(J) Cilia and Flagella:
Cilia (singular: cilium) and flagella (singular: flagellum) are hair-like protrusions from the cell membrane. Cilia are tiny structures that act like oars, causing the cell or the surrounding fluid to move. Flagella, on the other hand, are longer and are in charge of cell motility. Flagella are also seen in prokaryotic bacteria, however, they differ structurally from eukaryotic flagella.
An electron microscope examination of a cilium or flagellum reveals that they are encased in a plasma membrane. The axoneme, or core, severalseveralrotubules that run parallel to the long axis. Nine doublets of radially oriented peripheral microtubules and a pair of centrally positioned microtubules make up the axoneme. The 9+2 array refers to a specific arrangement of axonemal microtubules.
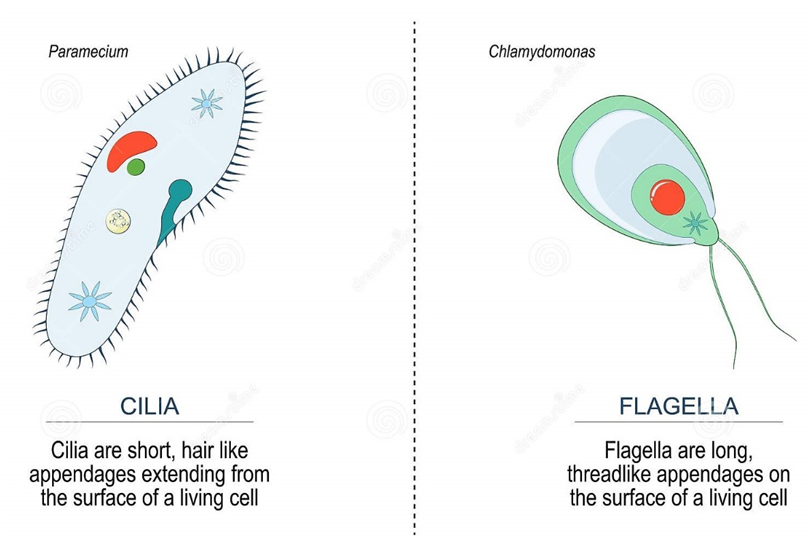
Figure 15: Difference between Cilia and Flagella.
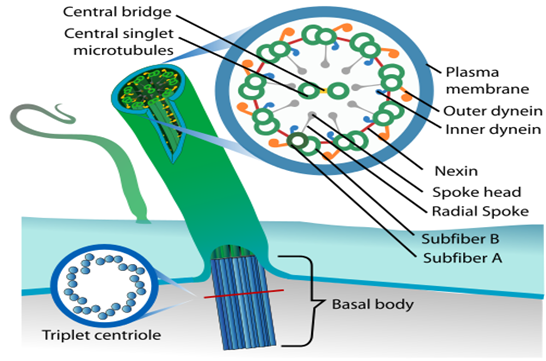
Figure 16: Structural arrangement in Cilia and Fagella.
The central tubules are linked by bridges and encased by a central sheath, which is linked to one of the tubules of each peripheral doublet by a radial spoke. There are nine radial spokes as a result. Linkers are also used to connect the peripheral doublets. The cilium and flagellum both emerge from the basal bodies, which are centriole-like structures.
(K) Centrosome and Centrioles:
A centrosome is an organelle that normally has two centrioles, which are cylindrical structures. Pericentriolar materials, which are amorphous, surround them. Both centrioles in a centrosome are perpendicular to one other and have a cartwheel-like arrangement. They are made up of nine tubulin protein peripheral fibrils that are uniformly spaced. Each peripheral fibril is made up of three triplets. The triplets next to it are also related. The hub, which is connected to tubules of the peripheral triplets by radial spokes comprised of protein, is located in the center section of the proximal region of the centriole. During cell division in animal cells, the centrioles create the basal body of cilia or flagella, as well as spindle fibers that give rise to the spindle apparatus.
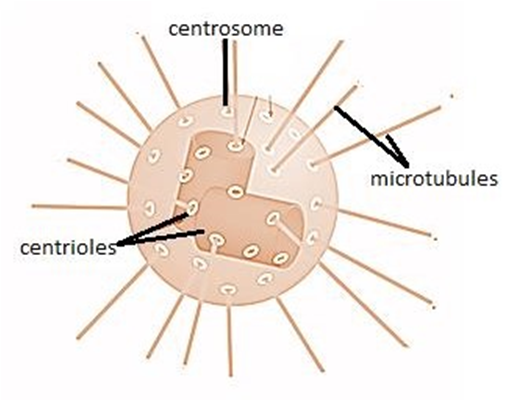
Figure 17: A centrosome and a centriole.
(L) Nucleus:
A nucleus is a membrane-bound organelle that governs and regulates the cell's functions (such as development and metabolism) and houses the structures that contain hereditary information, such as genes. Robert Brown was the first to characterize the nucleus in 1831. The nucleus is made up of a nuclear envelope, which is made up of two parallel membranes with a space between them termed the perinuclear space, according to electron microscopy. The nuclear membrane serves as a barrier between the contents of the nucleus and the cytoplasm. The outer membrane is normally connected to the endoplasmic reticulum and contains ribosomes. The nuclear envelope is punctured at several points by minute pores created by the merging of its two membranes.
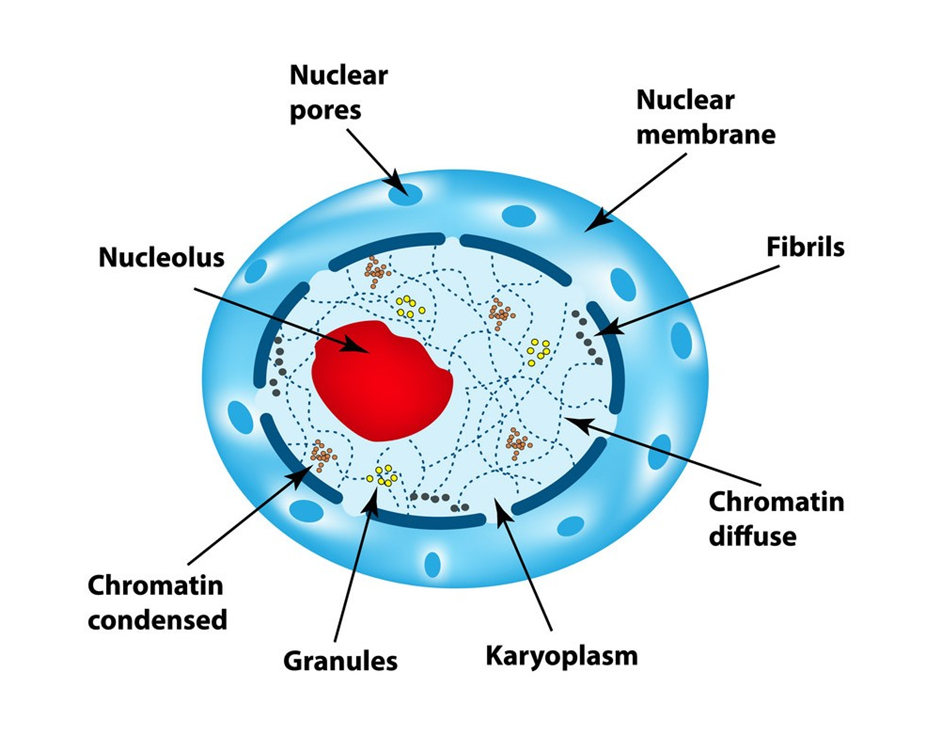
Figure 18: Nucleus
These nuclear pores are the conduits through which RNA and protein molecules travel between the nucleus and the cytoplasm in both directions. Although each cell normally has only one nucleus, variations in the number of nuclei are common. Nucleolus and chromatin are found in the nuclear matrix, also known as nucleoplasm. In the nucleoplasm, there are spherical structures called nucleoli. Because the nucleolus is not a membrane-bound structure, its contents are continuous with the rest of the nucleoplasm. It's where active ribosomal RNA synthesis happens. In cells that are actively synthesizing proteins, nucleoli are larger and more numerous.
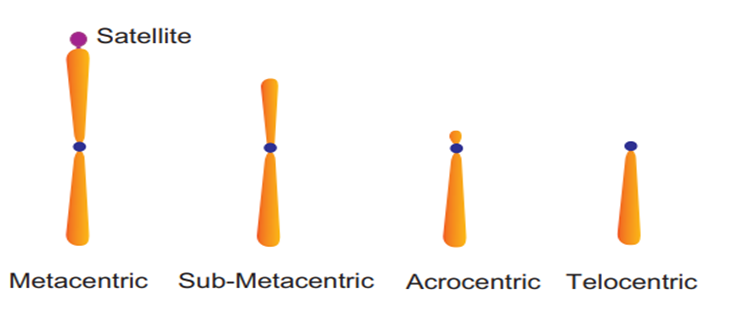
Figure 19: Types of Chromosomes.
The interphase nucleus is chromatin, which is a loose and unclear network of nucleoprotein fibers. Cells, on the other hand, reveal organized chromosomes in place of the nucleus at various phases of cell division. DNA, histones (basic proteins), non-histone proteins, and RNA are all found in chromatin. Each of the forty-six (twenty-three pairs) chromosomes in a single human cell has a two-meter-long strand of DNA.
Every chromosome (visible only in dividing cells) has a central constriction called the centromere, which is flanked by disc-shaped structures called kinetochores. A chromosome's centromere holds two chromatids.The chromosomes can be divided into four categories based on the position of the centromere. The central centromere of the metacentric chromosome divides the chromosome into two equal arms. The centromere of the sub-metacentric chromosome is located somewhat distant from the chromosome's center resulting in one shorter and one long arm. The centromere of an acrocentric chromosome is at the end, forming one extremely short and one extremely long arm, whereas a telocentric chromosome contains a terminal centromere.Occasionally, a few chromosomes will have non-staining secondary constrictions at the same spot. This appears to be a little component known as the satellite.
Microbodies:
Microbodies are membrane-bound minute vesicles that contain a variety of enzymes. Both plant and animal cells have them. Peroxisomes, glyoxysomes, glycosomes, and hydrogenosomes are all microbody organelles. Microbodies are particularly common in the liver and kidney of vertebrates.
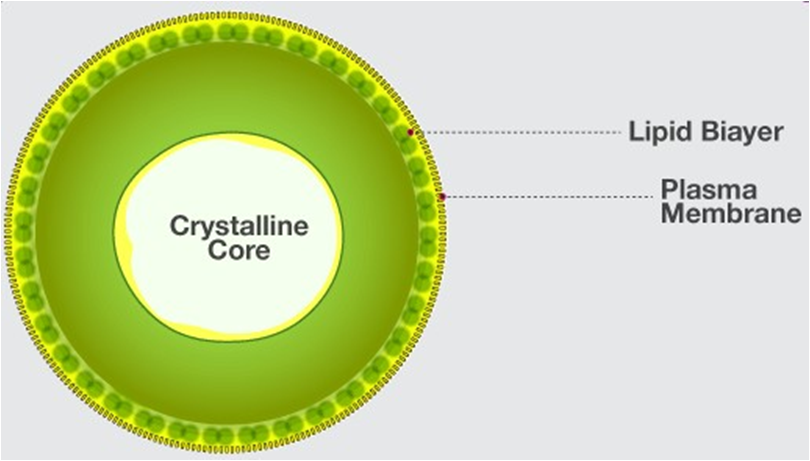
Figure 20.
How to Analyse Chemical Composition?
Chapter 9
Biomolecules
The biosphere has a vast variety of living species. Carbon, hydrogen, oxygen, and numerous other elements, as well as their respective contents, are acquired per unit mass of living tissue when an elemental analysis is done on plant tissue, animal tissue, or microbial paste. A similar list of elements will be obtained if the same analysis is performed on a piece of the earth's crust as an example of non-living stuff. A sample of live tissue contains all of the elements found in a sample of the earth's crust. However, a closer look reveals that any living organism has a higher relative quantity of carbon and hydrogen in relation to other elements than the earth's crust.
How to Analyse Chemical Composition?
A natural substance's elemental analysis reveals that it is made up of several elements such as carbon, hydrogen, oxygen, chlorine, and so on. Analytical procedures provide information on various organic and inorganic compounds, as well as their molecular formulas and structures. They also aid in the separation and purification of one component from another. Simple experiments can be used to determine the chemical composition of biomolecules. Crush and combine a piece of living tissue with an acid. We get two pieces after filtering it. The acid-soluble fraction of the filtrate is retained on the filter membrane, while the acid-insoluble fraction is retained on the filter membrane. This indicates that there are two or more substances with distinct characteristics within the tissues.Thousands of organic molecules have been discovered in the acid-soluble pool by scientists.
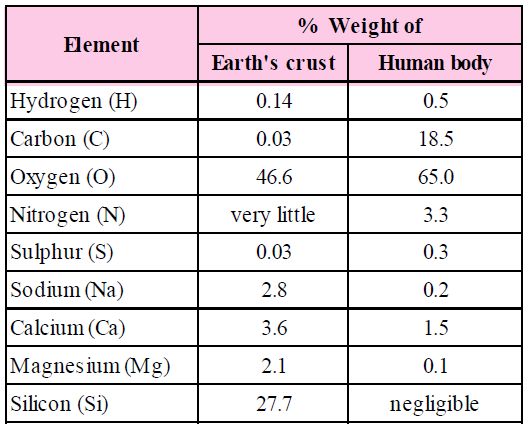
Constituents of Living Tissues
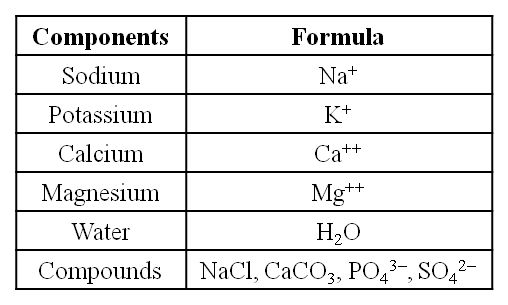
Take another piece and burn it till all the moisture in it is evaporated. When carbon compounds are burned, they are all oxidized. Inorganic substances such as calcium, magnesium, sulfate, phosphate, and others are formed in the tissue by the ash that has been left out.
Analytical techniques, when applied to a chemical, provide us with an estimate of its molecular formula and likely structure. 'Biomolecules' refers to all carbon compounds obtained from living tissues. All carbon-containing chemicals (organic compounds) found in living organisms are classified as biomolecules. They are organic substances found in living cells that have a role in the organism's maintenance and metabolic activities. Inorganic elements and compounds, on the other hand, are found in living beings. As a result, elemental analysis provides information on the composition of live tissues in terms of hydrogen, oxygen, chlorine, carbon, and other elements, whereas compound analysis provides information on the types of organic and inorganic constituents found in living tissues.
Functional groups such as aldehydes, ketones, aromatic molecules, and others can be detected in living tissues from a chemical standpoint. However, amino acids, nucleotide bases, fatty acids, and carbohydrates make up living tissues from a biological standpoint.
Amino acids are chemical molecules that have both an amino and an acidic group as substituents on the same carbon, the -carbon. As a result, they are known as -amino acids. They're methanes that have been replaced. The four valency locations are occupied by four substituent groups. Hydrogen, carboxyl group, amino group, and a variable group known as R group are the four groups. There are numerous amino acids in the R group due to its nature. However, there are only twenty varieties of those found in proteins. The R group in these proteinaceous amino acids could be a hydrogen (glycine), a methyl group (alanine), hydroxy methyl (serine), or something else entirely. The amino, carboxyl, and R functional groups make up the chemical and physical properties of amino acids.Acidic (e.g., glutamic acid), basic (lysine), and neutral (valine) amino acids are classified by the number of amino and carboxyl groups they contain.
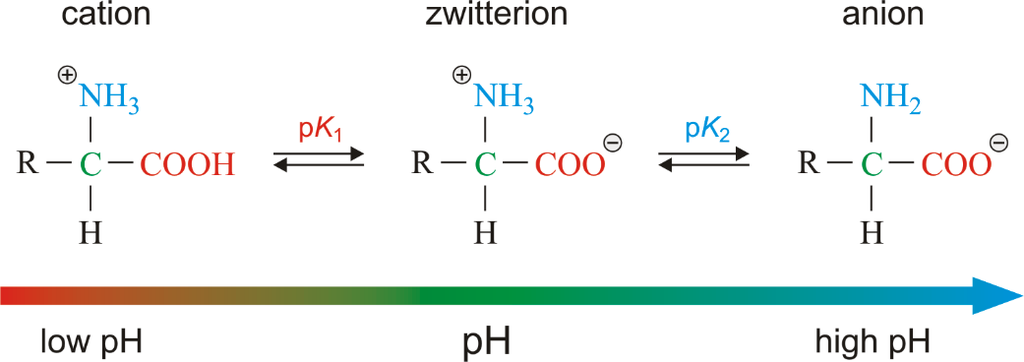
Similarly, aromatic amino acids exist (tyrosine, phenylalanine, tryptophan). The ionizability of the –NH2 and –COOH groups of amino acids is a unique feature. As a result, the structure of amino acids alters in different pH solutions.
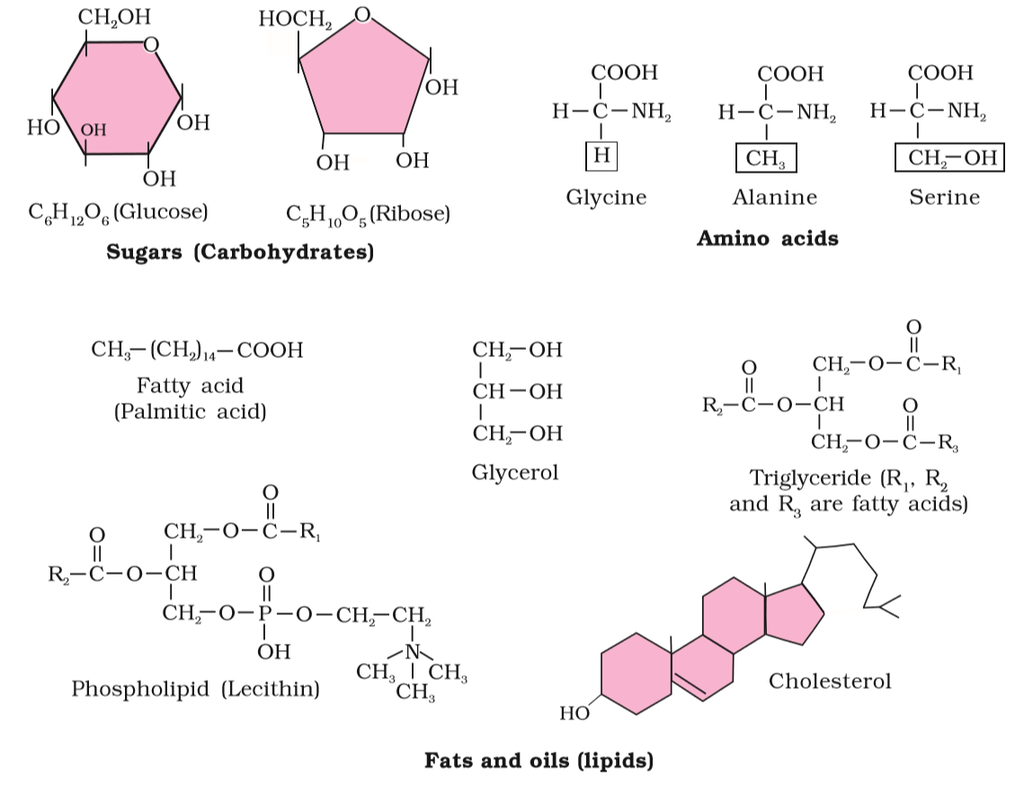
Figure 2 (a): Diagrammatic representation of small molecular weight organic compounds in living tissues
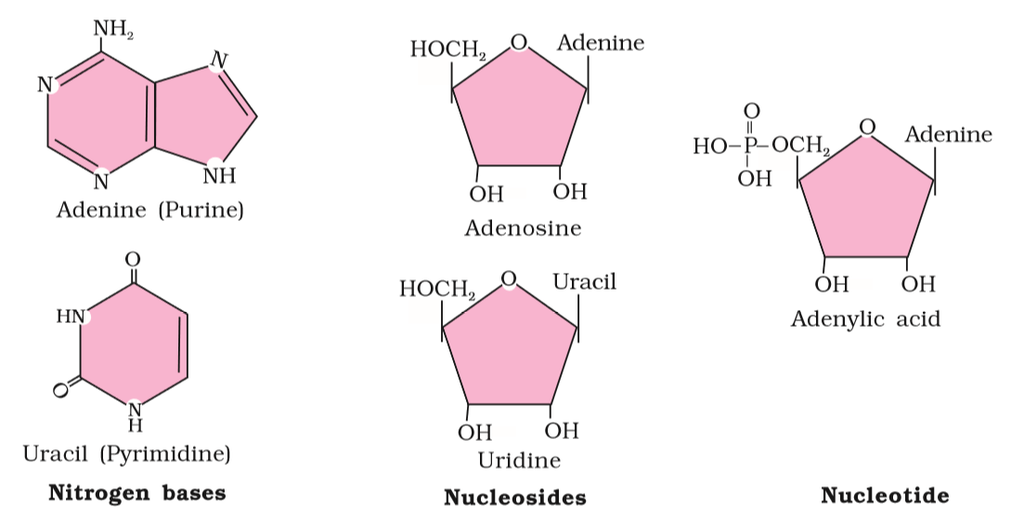
Figure 2(b): Diagrammatic representation of small molecular weight organic compounds in living tissues
Heterocyclic rings can be found in a variety of carbon compounds found in living beings. Adenine, guanine, cytosine, uracil, and thymine are examples of nitrogen bases. They're called nucleosides when they're found linked to sugar. Nucleotides are formed when a phosphate group is additionally esterified to the sugar. Nucleosides include adenosine, guanosine, thymidine, uridine, and cytidine. Nucleotides include adenylic acid, thymidylic acid, guanylic acid, uridylic acid, and cytidylic acid. Only nucleotides make up nucleic acids like DNA and RNA. DNA and RNA are two types of genetic material.
Primary and Secondary Metabolites
Primary and Secondary Metabolites
The most interesting component of chemistry is extracting thousands of small and large chemicals from live creatures, establishing their structure, and, if possible, synthesizing them. A list of biomolecules might contain thousands of organic chemicals, such as amino acids, carbohydrates, and other substances. These biomolecules can be referred to as metabolites. All of these kinds of chemicals can be found in animal tissues. Primary metabolites are what these are called. Thousands of additional substances termed secondary metabolites can be found in the plant, fungal, and microbial cells, such as alkaloids, flavonoids, rubber, essential oils, antibiotics, colored pigments, fragrances, gums, and spices. Secondary metabolites are what these are called.While primary metabolites have well-defined functions and roles in normal physiological processes, the role and functions of all secondary metabolites' in host organisms are currently unknown. Many of them, however, are beneficial to 'human welfare (e.g., rubber, drugs, spices, scents and pigments). Secondary metabolites play an important role in ecology.
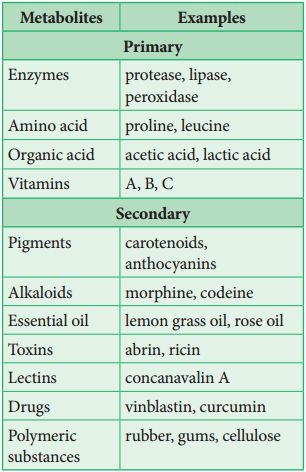
Biomacromolecules
Proteins, nucleic acids, polysaccharides, and lipids are the only organic molecules found in the acid-insoluble fraction. With the exception of lipids, these substances have molecular weights in the tens of thousands of Daltons or higher. Biomolecules, or chemical substances found in living beings, are divided into two categories for this reason. One, those which have molecular weights less than one thousand daltons and are commonly referred to as micromolecules or simply biomolecules whereas those which are discovered in the acid-insoluble fraction are dubbed macromolecules or biomacromolecules. All of the chemicals in the acid-soluble pool have one thing in common. They have molecular weights that range from 18 to 800 daltons (Da).
With the exception of lipids, the molecules in the insoluble fraction are polymeric. Lipids are small molecular weight substances that can be found in a variety of forms, including cell membranes and other membranes. When the cell structure is disturbed and the tissue ispulverized, the breakdown of cell membranes and other membranes results in the formation of vesicles that are not water-soluble. As a result, membrane fragments in the form of vesicles separate from the acid-insoluble pool, resulting in the macromolecular fraction. The cytoplasmic composition is generally represented by the acid-soluble pool. The acid-insoluble fraction contains macromolecules from the cytoplasm and organelles. They represent the total chemical composition of biological tissues when put together.In summation, it is observed that water is the most abundant component in living organisms upon characterizing the chemical makeup of living tissue from the standpoint of abundance and classifying them accordingly.
Carbohydrates
Carbohydrates
Carbohydrate is a group of organic compounds occurring in living tissues and foods in the form of starch, cellulose, and sugars. It is one of the three micronutrients via which a human body obtains energy. The properties of carbohydrate biology include carbon, hydrogen, and oxygen atoms at their chemical level. Cn(H2O)n is the generic formula for all carbohydrates. This formula is only valid for simple sugars, which are made up of the same amount of carbon and water.
There are two types of carbohydrates, simple and complex. This division is primarily based on their chemical structure along with their degree of polymerization.
Simple Carbohydrates: Simple carbohydrates carry one or two molecules of sugar. Such examples of carbohydrates are found abundantly in dairy products, refined sugar, etc. Since these carbohydrates do not comprise any fiber, vitamin, or mineral, they are regarded as empty calories. Simple carbohydrates can be further divided into three categories. These are as follows:
Monosaccharides: Carbohydrates consisting of one sugar molecule are called monosaccharides. Monosaccharides can be further classified based on the number of carbon atoms. These are trioses, tetroses, pentoses, hexoses, and heptoses. One of the most significant monosaccharides is glucose. The following are the two most frequent methods for preparing glucose. Sucrose is converted to glucose and fructose when it is cooked with dilute acid in an alcohol solution. From Starch, Glucose can also be made by hydrolyzing starch and boiling it with weak sulphuric acid at 393 degrees Fahrenheit under high pressure. Glucose, commonly known as dextrose and aldohexose, is abundant on the planet.
Disaccharides: Two monosaccharides combine to form a disaccharide. Sucrose, Lactose, and Maltose are some of the prime examples of this carbohydrate.
Oligosaccharides: Carbohydrates consisting of 2-9 monomers are classified as oligosaccharides.
The term “monosaccharide” refers to a carbohydrate derivative possessing a single carbon chain; “disaccharide” and “trisaccharide” refer to molecules containing two or three such monosaccharide units joined together by acetal or ketal linkages. “Oligosaccharide” and “polysaccharide” refer to larger such aggregates, with “a few” and many monosaccharide units, respectively. Current usage seems to draw the distinction between “few” and many at around 10 units.
Complex Carbohydrate: Complex carbohydrates are made up of two or more molecules of sugar. Such carbohydrates are found abundantly in food items like corn, lentils, peanuts, beans, etc. Complex carbohydrates are also known as polysaccharides as they are formed due to polymerization.
Polysaccharides are another macromolecule family found in the acid-insoluble pellet. Polysaccharides are lengthy sugar chains. They're threads (literally, cotton threads) made up of various monosaccharides as building blocks. cellulose, for example, is a polymeric polysaccharide made up of only one type of monosaccharide, glucose. Cellulose is a homopolymer, which means it is made up of only one type of molecule. Starch is a type of this that is found in plant tissues as a source of energy. Glycogen is a different type of carbohydrate found in animals. Inulin is a fructose polymer. The right end of a polysaccharide chain (such as glycogen) is known as the reducing end, while the left end is known as the non-reducing end. It has branches. Secondary helical structures are formed by starch.In fact, the helical part of starch can retain I2 molecules. The colour of starch-I2 is blue. Because cellulose lacks complex helices, it is unable to retain I2.
Cellulose is the main component of plant cell walls. Cellulosic paper is created from plant pulp and cotton fibre. In nature, there exist more complicated polysaccharides. Amino-sugars and chemically modified sugars are used as building blocks (e.g., glucosamine, N-acetyl galactosamine, etc.). Arthropod exoskeletons, for example, contain a complex polymer called chitin. The majority of these complex polysaccharides are homopolymers.
Amino Acids
Amino Acids
Amino acids are chemical molecules with amino and carboxylate functional groups as well as a side chain that is unique to each amino acid. Amino Acids are chemical substances that combine to produce proteins, which is why they are known as the building blocks of proteins. These biomolecules have a role in a variety of biological and chemical functions in the human body and are essential for human growth and development. There are around 300 amino acids found in nature. Amino acids' general features include:
1. A high melting and boiling point.
2. Amino acids are crystalline solids that are white in color.
3. Few amino acids have a pleasant, tasteless, or bitter flavour.
4. The majority of amino acids are water-soluble and insoluble in organic solvents.
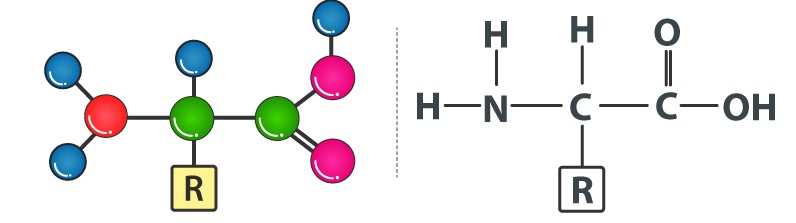
There are 20 amino acids found in nature, all of which have the same structural features: an amino group (-NH3+), a carboxylate group (-COO-), and a hydrogen-bonded to the same carbon atom. Their side-chain, known as the R group, distinguishes them from one another. The - carbon of each amino acid is connected to four distinct groups namely: Amino group, COOH, Hydrogen atom, and Sidechain.
Amino acids have a general structure which can be represented as:
Our bodies can easily produce a few non-essential amino acids out of a total of 20 amino acids. Alanine, asparagine, arginine, aspartic acid, glutamic acid, cysteine, glutamine, proline, glycine, serine, and tyrosine are examples of these amino acids.
Besides these, there are nine other amino acids that are extremely important because our bodies cannot make them. Isoleucine, histidine, lysine, leucine, phenylalanine, tryptophan, methionine, threonine, and valine are examples of important amino acids.
Amino acids are essential for a variety of biological and chemical tasks in our bodies, including tissue construction and repair, enzyme production and activity, food digestion, molecule transportation, and so on. Only a few amino acids can be synthesized by our bodies, thus the rest, known as essential amino acids, must be obtained from protein-rich foods in our daily diet. Plant-based foods with high levels of amino acids include broccoli, beans, beets, pumpkin, cabbage, almonds, dry fruits, chia seeds, oats, peas, carrots, cucumber, green leafy vegetables, onions, soybeans, whole grain, peanuts, legumes, lentils, and so on. Apples, bananas, berries, figs, grapes, melons, oranges, papaya, pineapple, and pomegranates are high in amino acids.Dairy products, eggs, seafood, poultry, beef, pork, and other animal products are examples of other animal goods.
Functions of Essential Amino acids
1. Phenylalanine helps in maintaining a healthy nervous system and in boosting memory power.
2. Valine acts as an important component in promoting muscle growth.
3. Threonine helps in promoting the functions of the immune system.
4. Tryptophan is involved in the production of vitamin B3 and serotonin hormones. This serotonin hormone plays a vital role in maintaining our appetite, regulating sleep and boosting our moods.
5. Isoleucine plays a vital role in the formation of hemoglobin, stimulating the pancreas to synthesize insulin, and transporting oxygen from the lungs to the various parts.
6. Methionine is used in the treatment of kidney stones, maintaining healthy skin and also used in controlling invade of pathogenic bacteria.
7. Leucine is involved in promoting protein synthesis and growth hormones.
8. Lysine is necessary for promoting the formation of antibodies, hormones, and enzymes and in the development and fixation of calcium in bones.
9. Histidine is involved in many enzymatic processes and in the synthesizing of both red blood cells (erythrocytes) and white blood cells (leukocytes).
Functions of Non-Essential Amino acids
1. Alanine functions by removing toxins from our body and in the production of glucose and other amino acids.
2. Cysteine acts as an antioxidant and provides resistance to our body; it is important for making collagen. It affects the texture and elasticity of the skin
3. Glutamine promotes a healthy brain function and is necessary for the synthesis of nucleic acids – DNA and RNA.
4. Glycine is helpful in maintaining the proper cell growth, and its function, and it also plays a vital role in healing wounds. It acts as a neurotransmitter.
5. Glutamic acid acts as a neurotransmitter and is mainly involved in the development and functioning of the human brain.
6. Arginine helps in promoting the synthesis of proteins and hormones, detoxification in the kidneys, healing wounds, and maintaining a healthy immune system.
7. Tyrosine plays a vital role in the production of the thyroid hormones -T3 and T4, in synthesizing a class of neurotransmitters and melanin, which are natural pigments found in our eyes, hair, and skin.
8. Serine helps in promoting muscle growth and in the synthesis of immune system proteins.
9. Asparagine is mainly involved in the transportation of nitrogen into our body cells, formations of purines and pyrimidine for the synthesis of DNA, the development of the nervous system and improving our body stamina.
10. Aspartic acid plays a major role in metabolism and in promoting the synthesis of other amino acids.
11. Proline is mainly involved in the repairing of the tissues and the formation of collagen, preventing the thickening and hardening of the walls of the arteries (arteriosclerosis) and in the regeneration of new skin.
Proteins
Proteins
Polypeptides are the building blocks of proteins. They are peptide bonds that connect linear chains of amino acids. A polymer of amino acids makes up each protein. A protein is a heteropolymer, not a homopolymer because there are 20 different types of amino acids. A homopolymer is made up of only one type of monomer that repeats 'n' times. Specific amino acids are necessary for human health and must be obtained through our food. As a result, necessary amino acids are obtained from food proteins. As a result, amino acids can be classified as either essential or non-essential. The latter are those that our bodies can produce, whereas essential amino acids must be obtained through our diet.Proteins have a variety of activities in living creatures, including transporting nutrients across cell membranes, fighting pathogenic organisms, acting as hormones, and enzymatic reactions. The most abundant protein in the animal kingdom is collagen, and the most abundant protein in the biosphere is Ribulose bisphosphate Carboxylase-Oxygenase (RuBisCO).
Structure of Proteins:
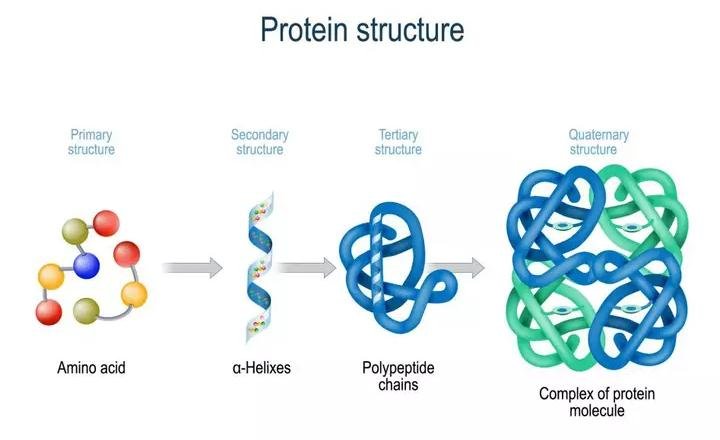
Proteins are heteropolymers made up of strings of amino acids, as previously stated. In different situations, the structure of molecules means different things. Physicists visualize molecular structures in three dimensions, while biologists explain protein structure on four levels. The primary structure of a protein is the sequence of amino acids, or the positional information in a protein — which amino acid is the first, which is the second, and so on.A protein can be visualized as a line, with the initial amino acid on the left end and the last amino acid on the right.
N-terminal amino acid is another name for the first amino acid. The C-terminal amino acid is the last one in the chain. As an extended stiff rod, a protein thread does not exist throughout. The thread is folded into a helix shape (similar to a revolving staircase). Naturally, only a piece of the protein thread is organized in a helix shape. Only right-handed helices are found in proteins. In what is known as the secondary structure, various portions of the protein thread are folded into other configurations.
Furthermore, the lengthy protein chain folds in on itself like a hollow woollen ball, forming the tertiary structure. This allows us to see a protein in three dimensions. Proteins' tertiary structure is required for many of their biological functions. Some proteins are made up of several polypeptides and subunits. The architecture of a protein, also known as the quaternary structure of a protein, is the way these individual folded polypeptides or subunits are arranged with regard to one other (e.g., a linear string of spheres, spheres placed one upon another in the form of a cube or plate, etc.).Adult human haemoglobin consists of 4 subunits. Two of these are identical to each other. Hence, two subunits of α type and two subunits of β type together constitute the human haemoglobin (Hb).
Nature of bond linking monomers in a polymer
A peptide bond is produced when the carboxyl (-COOH) group of one amino acid combines with the amino (-NH2) group of the next amino acid with the elimination of a water molecule in a polypeptide or protein (the process is called dehydration). Individual monosaccharides in a polysaccharide are joined by a glycosidic bond. Dehydration also contributes to the formation of this link. Two carbon atoms from two neighboring monosaccharides make a peptide bond. A phosphate moiety of a nucleic acid connects the 3'-carbon of one sugar of one nucleotide to the 5'-carbon of the sugar of the next nucleotide. An ester link exists between the phosphate and the sugar's hydroxyl group.The phosphodiester bond is named after the fact that there is one such ester bond on each side.
Lipids
Lipids
Lipids are organic molecules containing hydrogen, carbon, and oxygen atoms that provide the structural and functional foundation for living cells. Because water is a polar molecule, these organic compounds are nonpolar molecules that are only soluble in nonpolar solvents and insoluble in water. These molecules are produced in the human liver and can be found in the oil, butter, whole milk, cheese, fried foods, and some red meats.
Structure of Lipids
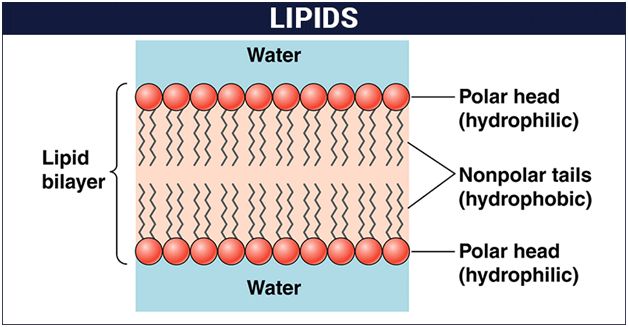
Lipids are fatty acid polymers with a long, non-polar hydrocarbon chain and a short polar area that contains oxygen.Lipids are often insoluble in water. It's possible that they're just simple fatty acids. A carboxyl group is connected to the R group in a fatty acid. The R group could be methyl (–CH3), ethyl (–C2H5), or a combination of the two (1 carbon to 19 carbons). Palmitic acid, for example, comprises 16 carbons, including the carboxyl carbon. Arachidonic acid, including carboxyl carbon, has 20 carbon atoms.Saturated fatty acids have no double bonds, while unsaturated fatty acids have one or more C=C double bonds. Glycerol, which is trihydroxy propane, is another simple lipid. Glycerol and fatty acids are found in many lipids. The fatty acids are esterified with glycerol. Monoglycerides, diglycerides, and triglycerides are the three types of lipids. Based on their melting point, these are also known as fats and oils. Oils (e.g., gingely oil) have a lower melting point and hence remain as oil in the winter. Phosphorus and a phosphorylated organic molecule are found in some lipids. Phospholipids are what they're called. They're located in the membranes of cells. One example is lecithin. Lipids with more complicated structures are found in some tissues, particularly brain tissues.
Listed below are some important characteristics of Lipids.
1. Lipids are oily or greasy nonpolar molecules, stored in the adipose tissue of the body.
2. Lipids are a heterogeneous group of compounds, mainly composed of hydrocarbon chains.
3. Lipids are energy-rich organic molecules, which provide energy for different life processes.
4. Lipids are a class of compounds characterized by their solubility in nonpolar solvents and insolubility in water.
5. Lipids are significant in biological systems as they form a mechanical barrier dividing a cell from the external environment known as the cell membrane.
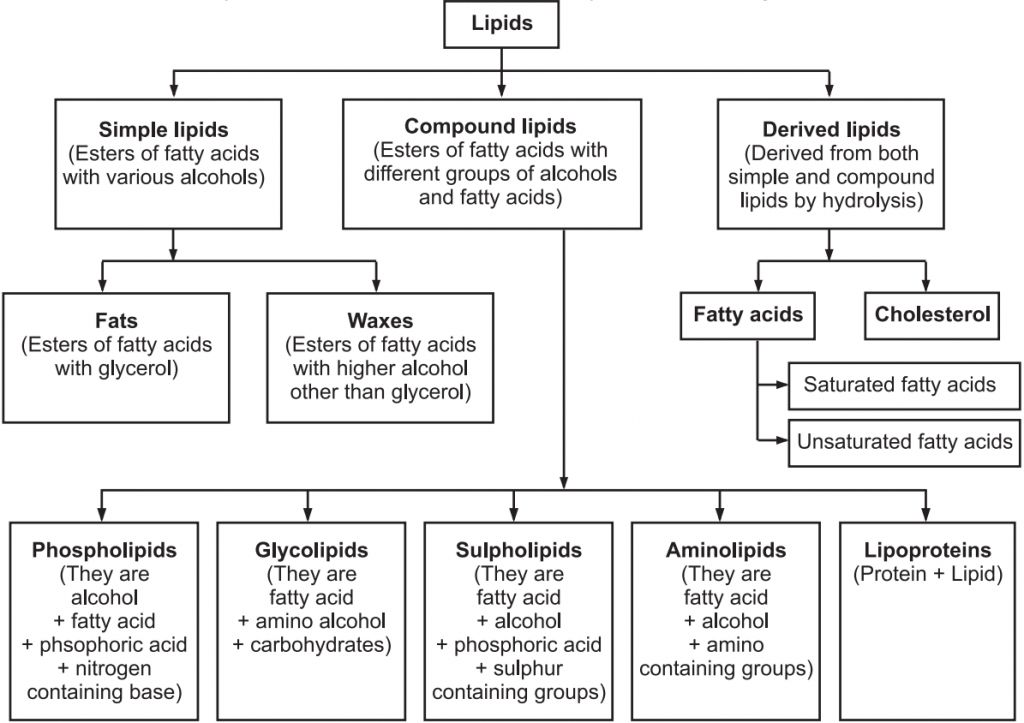
Classification of Lipids:
Lipids serve a critical role in our bodies. They are a component of the cell membrane's structure. They aid in the production of hormones and provide energy to our bodies. They aid in appropriate meal digestion and absorption. If we eat them in the right amounts, they constitute a nutritious element of our diet. They play a vital function in signaling as well.
Nucleic Acids
Nucleic Acids
The nucleic acid is another form of macromolecule found in the acid-insoluble portion of any living tissue. Polynucleotides are a type of nucleotide. They make up the real macromolecular fraction of any living tissue or cell, together with polysaccharides and polypeptides. A nucleotide is the building block of nucleic acids. A nucleotide is made up of three chemically different parts. A heterocyclic molecule is one, a monosaccharide is another, and phosphoric acid or phosphate is the third. The nitrogenous bases adenine, guanine, uracil, cytosine, and thymine are heterocyclic molecules in nucleic acids. The purines Adenine and Guanine are substituted, with the pyrimidines Thymine and Cytosine. Purine and pyrimidine are the components for the skeletal heterocyclic ring.Polynucleotides include either ribose (a monosaccharide pentose) or 2' deoxyribose as a sugar. Deoxyribonucleic acid (DNA) is a nucleic acid that contains deoxyribose, whereas ribonucleic acid (RNA) contains ribose sugar.
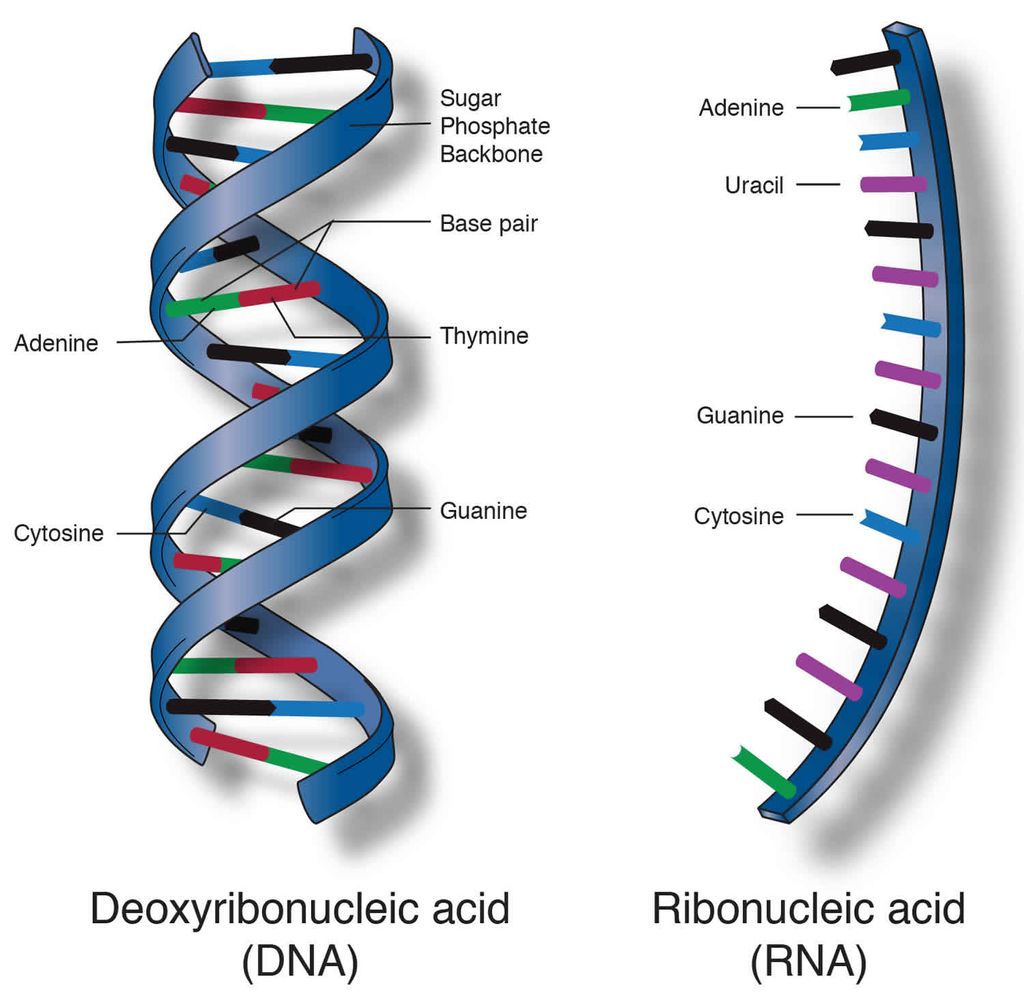
Secondary structures in nucleic acids come in a range of shapes and sizes. The famous Watson - Crick Model, for example, is one of DNA's secondary structures. According to this idea, DNA is a double helix. Polynucleotide strands are antiparallel, meaning they run in opposite directions. The sugar-phosphate-sugar chain makes up the backbone. The nitrogen bases are positioned perpendicular to the backbone but on the inside. One strand's A and G must base pair with the other strand's T and C, respectively. Between A and T, there are two hydrogen bonds, while between G and C, there are three hydrogen bonds.
Each thread resembles a spiral staircase. A pair of bases represent each stage of the ascent. The strand rotates 36 degrees with each climbing step. Ten steps or ten base pairs would be required to complete a full turn of the helical strand. It would be a 34 pitch. The increase would be 3.4 per base pair. B-DNA is a type of DNA that has the characteristics listed above. More than a dozen kinds of DNA named after English alphabets with unique structural properties are known to exist.
Concept of Metabolism and living state
Concept of metabolism and living state
Thousands of organic chemicals can be found in living species, whether they be bacteria, protozoa, plants, or animals. These substances or biomolecules exist at specific concentrations (mols/cell, mols/litre, and so on). The discovery that all biomolecules have a turnover was one of the most significant discoveries ever made. This means they're continually changing into and out of different biomolecules. Chemical reactions occur frequently in living organisms, breaking and making this possible. Metabolism refers to the sum of all these chemical events. The change of biomolecules occurs in each of the metabolic processes.
Removal of carbon di oxide from amino acids, resulting in an amine, removal of amino group in a nucleotide base, hydrolysis of a glycosidic bond in a disaccharide, and so on are some examples of metabolic transformations. There are tens of thousands of examples like this. The majority of these metabolic events are always related to other reactions and do not occur in isolation. In other words, metabolites are changed into one another through metabolic pathways, which are a set of related reactions. These metabolic pathways are comparable to city traffic in terms of complexity. There are two types of pathways: linear and circular. There are traffic crossroads where these paths cross one other.Like car traffic, the flow of metabolites through the metabolic pathway has a set rate and direction. The dynamic state of bodily constituents refers to this metabolite flux. What matters most is that this interconnected metabolic traffic runs smoothly and without a single reported hiccup under normal circumstances. Every chemical reaction in these metabolic pathways is a catalysed reaction, which is another distinguishing trait. In biological systems, there is no metabolic conversion that is not catalysed. Even the physical process of CO2 dissolving in water is a catalysed reaction in biological systems. Proteins are also catalysts that speed up the tempo of a metabolic conversation. Enzymes are the name given to these catalytic proteins.
Metabolic basis for living
Metabolic pathways can either lead to a more complex structure from a simpler structure (e.g., acetic acid becomes cholesterol) or a simpler structure from a complex structure (e.g., acetic acid becomes cholesterol) (for example, glucose becomes lactic acid in our skeletal muscle). The former are referred to as biosynthetic or anabolic pathways. The latter are referred to as catabolic pathways since they involve degradation. Anabolic pathways, use a lot of energy. Energy is required to assemble a protein from amino acids. Catabolic pathways, on the other hand, result in the release of energy. When glucose is metabolized to lactic acid in skeletal muscles, for example, energy is released. Glycolysis is a metabolic mechanism that takes glucose and converts it to lactic acid in ten stages.
Living organisms have figured out how to capture the energy released during breakdown and store it as chemical bonds. This bond energy is used as needed for biosynthetic, osmotic, and mechanical tasks that we do. The bond energy in a molecule called adenosine triphosphate is the most essential kind of energy currency in living systems (ATP).
The living state
Tens and thousands of chemical compounds in a living organism, otherwise called metabolites, or biomolecules, are present at concentrations characteristic of each of them. For example, the blood concentration of glucose in a normal healthy individual is 4.5-5.0 mM. The most important fact of biological systems is that all living organisms exist in a steady-state characterized by concentrations of each of these biomolecules. These biomolecules are in metabolic flux. Any chemical or physical process moves spontaneously to equilibrium. The steady state is a non-equilibrium state. One should remember from physics that systems at equilibrium cannot perform work. As living organisms work continuously, they cannot afford to reach equilibrium.
Thus, to be able to conduct work, the living state is a non-equilibrium steady-state; the living process is a constant endeavour to avoid slipping into equilibrium. This is accomplished by the use of energy. Metabolism is the process by which energy is produced. As a result, the terms "living state" and "metabolism" are interchangeable. There can be no living condition without metabolism.
Enzymes
Enzymes
Proteins make up almost all enzymes. Some nucleic acids have enzyme-like properties. Ribozymes are the name for these proteins. A line diagram can be used to represent an enzyme. An enzyme, like any other protein, has a primary structure, which is the amino acid sequence. Secondary and tertiary structures are present in enzymes, as they are in other proteins. When you look at a tertiary structure, you'll observe that the protein chain's backbone folds in on itself, the chain crisscrosses itself, and many crevices or pockets form. The 'active site' is one such pocket. An enzyme's active site is a crevice or pocket that the substrate fits into. As a result, enzymes catalyze processes at a rapid pace through their active site.
In many aspects, enzyme catalysts differ from inorganic catalysts, but one key distinction stands out. At high temperatures and pressures, inorganic catalysts perform efficiently, but enzymes are destroyed at high temperatures (say, above 40°C). Enzymes extracted from organisms that dwell in severe heat (e.g., hot vents and sulphur springs) are stable and keep their catalytic power even at extreme temperatures (up to 80°-90°C). The capacity of such enzymes extracted from thermophilic organisms to maintain their thermal stability is thus critical.
Chemical reactions
Chemical compounds go through two sorts of transformations. A physical change is merely a change in shape that does not involve the breaking of connections.This is a bodily function. A change in state of matter is another physical process, such as when ice melts into water or when water becomes vapour. These are physical processes as well. A chemical reaction, on the other hand, occurs when bonds are broken and new bonds are generated during transformation. For example
Ba (OH)2+ H2SO4 ® BaSO4 + 2H2O
is a chemical process that takes place in an inorganic state. The conversion of starch to glucose is also an organic chemical reaction. The amount of product created per unit time is referred to as the rate of a physical or chemical process. It can be written as:
rate=dP/ dT
If the direction is indicated, rate is also known as velocity. Temperature, among other things, affects the rates of physical and chemical processes. For every 10°C change in either direction, the rate doubles or declines by half, according to a common rule of thumb. Catalyzed reactions proceed at a much faster rate than uncatalyzed reactions. For every 10°C change in either direction, the rate of enzyme catalysed reactions would be substantially larger than the same but uncatalyzed reaction. Catalyzed reactions proceed at a much faster rate than uncatalyzed reactions. When enzyme-catalyzed reactions are detected, the rate is much higher than when the identical process is not catalysed. As an example,
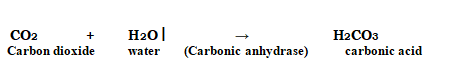
In the absence of any enzyme, this reaction is extremely sluggish, producing just around 200 molecules of H2CO3 per hour. The reaction speeds up substantially when carbonic anhydrase, an enzyme found in the cytoplasm, is used, with roughly 600,000 molecules generated every second. The enzyme has sped up the reaction rate by a factor of ten million. The power of enzymes is very amazing! Thousands of different enzymes exist, each catalysing a different chemical or metabolic reaction. A metabolic route is a multistep chemical reaction in which each step is catalysed by the same enzyme complex or separate enzymes. As an example,
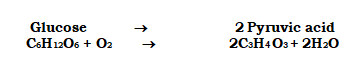
is a metabolic pathway in which glucose is converted to pyruvic acid via 10 enzyme-catalyzed chemical events. At this point, you should be aware that this metabolic pathway, when supplemented with one or two additional processes, produces a wide range of metabolic end products. Lactic acid is produced in skeletal muscle under anaerobic circumstances. Pyruvic acid is generated under typical aerobic circumstances. The similar route leads to the generation of ethanol in yeast during fermentation (alcohol). As a result, different products are possible depending on the circumstances.
How do Enzymes bring about such High Rates of Chemical Conversions?
The concept of an active site is already known. A reaction is referred to as a chemical or metabolic change. A'substrate' is the chemical that is transformed into a product. As a result, enzymes, which are three-dimensional proteins with a 'active site,' convert a substrate (S) into a product (P). This can be represented symbolically as
![]()
Within a specific cleft or pocket, the substrate 'S' must bind the enzyme at its'active site.' The substrate must diffuse into the 'active site.' As a result, the creation of a 'ES' complex is required. The letter E stands for enzyme. This intricate structure is a one-time occurrence. A new structure of the substrate called the transition state structure is created when the substrate is attached to the enzyme active site. The product is quickly removed from the active site after the intended bond breaking/making is completed. In other words, the substrate's structure is changed into the product's structure (s). This transformation must pass via a structure known as a transition state structure.Between the stable substrate and the result, there could be many additional 'altered structural states.' The notion that all other intermediate structural states are unstable is implicit in this assertion. Stability has to do with the molecule's or structure's energy status.
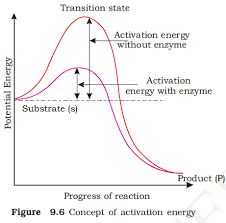
The potential energy content is shown by the y-axis. The x-axis depicts the structural transformation or states as they proceed through the 'transition stage.' There would be two things that you would notice. The difference in energy levels between S and P. When 'P' is less than 'S,' the reaction is exothermic. It is not necessary to provide energy (through heating) in order to manufacture the product. Regardless of whether the reaction is exothermic or spontaneous or endothermic or energy-demanding, the 'S' must pass through a significantly higher energy level or transition state. 'Activation energy' is the difference between the average energy content of 'S' and that of this transition state. Enzymes gradually break down this energy barrier, allowing the 'S' to become 'P' more easily.
Nature of Enzyme Action
In order to create a highly reactive enzyme-substrate complex (ES), each enzyme (E) has a substrate (S) binding site in its molecule. This complex has a brief half-life and dissociates into its product(s) P and the unmodified enzyme, with the enzyme-product complex forming in the middle (EP). Catalysis requires the creation of the ES complex.
E+S → ES → EP → E+P
An enzyme's catalytic cycle can be broken down into the following steps:
1. The substrate first binds to the enzyme's active site, fitting into the active site.
2. The enzyme's shape changes as a result of the substrate's binding, causing it to fit more tightly around the substrate.
3. The enzyme's active site, which is now in close proximity to the substrate, breaks the substrate's chemical bonds, forming a new enzyme-product complex.
4. The reaction products are released by the enzyme, and the free enzyme is ready to bind to another molecule of the substrate and repeat the catalytic cycle.
Factors Affecting Enzyme Activity
A change in the circumstances can impact the activity of an enzyme by altering the protein's tertiary structure. Temperature, pH, changes in substrate concentration, and the binding of certain molecules that affect its activity are examples of these.
Temperature and pH: Enzymes typically operate within a narrow temperature and pH range. The optimum temperature and pH for each enzyme are defined as the temperature and pH at which the enzyme is most active. Both below and above the ideal value, activity decreases. Because proteins are denatured by heat, low temperatures maintain the enzyme in a temporarily inactive state, but high temperatures eliminate enzymatic function.
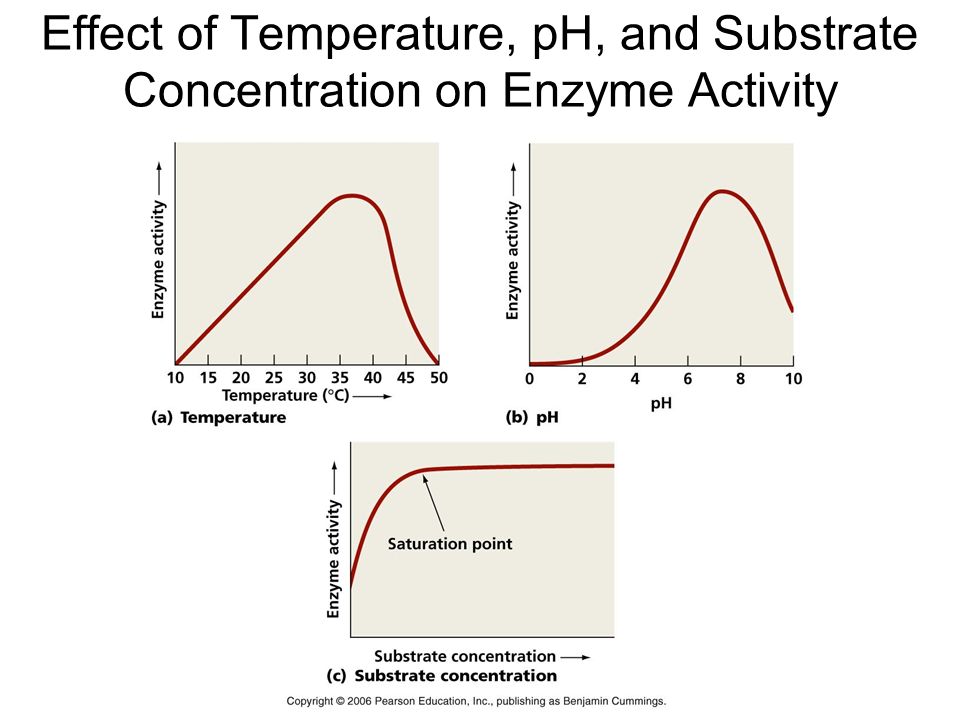
Substrate Concentration: At first, the enzymatic reaction's velocity increases as the substrate concentration rises. The reaction eventually achieves a maximum velocity (Vmax) that isn't exceeded by any further increase in substrate concentration. This is because there are fewer enzyme molecules than substrate molecules, and there are no free enzyme molecules to interact with the additional substrate molecules after these molecules have been saturated. An enzyme's activity is also affected by the presence of certain molecules that bind to it. The process of inhibition occurs when a chemical binds to an enzyme, and the chemical is referred to as an inhibitor.Competitive inhibitors are those that have a chemical structure that closely mimics that of the substrate and inhibits the enzyme's activity. Because of its structural similarity to the substrate, the inhibitor competes with it for the enzyme's substrate binding site. As a result, the substrate is unable to attach, and the enzyme's activity is reduced, as seen in the inhibition of succinic dehydrogenase by malonate, which is structurally similar to the substrate succinate. Competitive inhibitors like these are frequently employed to combat bacterial infections.
Classification and Nomenclature of Enzymes
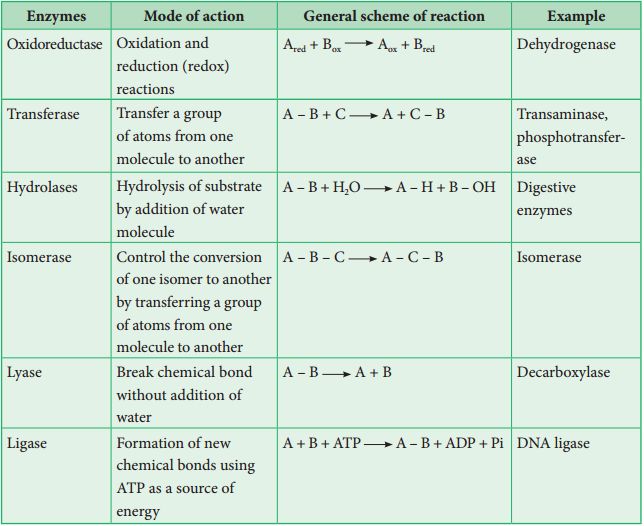
There have been thousands of enzymes identified, isolated, and analyzed. The majority of these enzymes have been divided into groups depending on the kind of reactions that they catalyze. Enzymes are classified into six classes, each having four to thirteen subclasses, and given a four-digit number.
Co-factors
Enzymes are polypeptide chains made up of one or more polypeptide chains. However, in other situations, non-protein elements known as cofactors are coupled to the enzyme in order for it to be catalytically active. The apoenzyme refers to the protein part of the enzyme in these cases. Prosthetic groups, co-enzymes, and metal ions are all examples of cofactors. Prosthetic groups are chemical molecules that are strongly attached to the apoenzyme, distinguishing them from other cofactors. The prosthetic group haem is a component of the active site of enzymes like peroxidase and catalase, which catalyze the breakdown of hydrogen peroxide to water and oxygen.Co-enzymes are organic substances as well, although their relationship with the apoenzyme is very temporary, occurring most often during catalysis. Co-enzymes are also used as co-factors in a variety of enzyme-catalyzed processes. Many coenzymes contain vitamins as important chemical components; for example, the coenzymes nicotinamide adenine dinucleotide (NAD) and NADP contain the vitamin niacin. Metal ions that create coordination bonds with side chains in the active site while also forming one or more coordination bonds with the substrate are required for the activity of a number of enzymes, for example, zinc is a cofactor for the proteolytic enzyme carboxypeptidase. When a co-factor is removed from an enzyme, its catalytic activity is lost, indicating that they play an important part in the enzyme's catalytic activity.
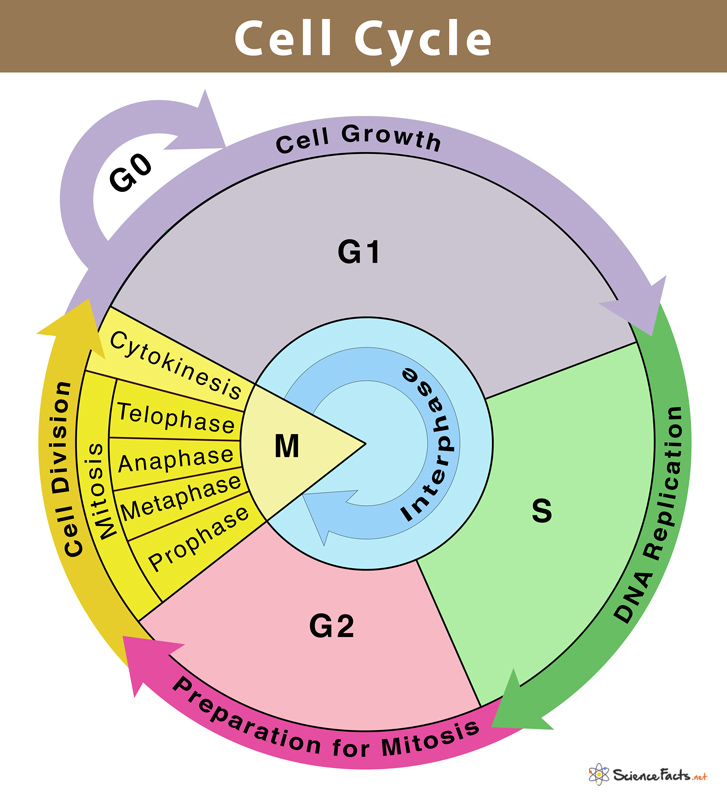
Phases of the Cell Cycle
Phases of Cell Cycle
Human cells in culture provide an example of a normal eukaryotic cell cycle. Every 24 hours or so, these cells divide once. The length of the cell cycle can, however, differ from organism to organism and from cell type to cell type. For instance, yeast may complete the cell cycle in about 90 minutes.
Interphase and M Phase(Mitosis phase) are the two fundamental phases of the cell cycle.
Interphase
A cell spends the majority of its time in what is known as interphase, where it develops, duplicates its chromosomes, and gets ready to divide. The cell then exits interphase, goes through mitosis, and finishes dividing. The phases of interphase are G1 phase (cell growth), S phase (DNA synthesis), and G2 phase (cell growth). The mitotic phase, which consists of mitosis and cytokinesis and produces two daughter cells, begins after interphase. In the cell cycle, the interphase is a protracted resting phase during which , DNA is replicated, RNA is synthesised, and proteins are produced.
The transitional period between mitosis and the start of DNA replication is known as the G1 phase. The cell is metabolically active and continues to develop during the G1 phase but does not duplicate its DNA. The time when DNA synthesis or replication occurs is known as the "S" phase. The amount of DNA in each cell doubles throughout this period. DNA grows from 2C (the initial amount) to 4C (the final amount). However, the number of chromosomes does not grow; if the cell had diploid or 2n chromosomes at G1, the number of chromosomes continues to be 2n even after S phase.In animal cells, the centriole doubles in the cytoplasm and DNA replication starts in the nucleus during the S phase. Proteins are created during the G2 phase as cells continue to expand in preparation for mitosis.Thus interphase is vital in the cell cycle as it permits the cell to grow and develop into a mature cell before it is able to reproduce.
Heart cells are one type of cell that does not appear to divide in adult animals, and many other cells only sporadically divide when it is necessary to replenish cells lost to damage or cell death. These cells leave the G1 phase and enter the quiescent stage (G0), which is an inactive phase of the cell cycle. While still metabolically active, cells in this stage no longer divide unless specifically instructed to do so by the organism. Only the diploid somatic cells in animals undergo mitotic cell division. In contrast, both haploid and diploid cells in the plants can exhibit mitotic divisions.
M Phase
It is the most dramatic phase of the cell cycle and involves a significant reorganisation of almost all cell components. It is also known as equational division since both the parent and progeny cells have the same number of chromosomes. Although mitosis has been conveniently split into four stages of nuclear division, it is crucial to realise that cell division is a progressive process, and extremely obvious distinctions between different stages cannot be made. Two processes make up the M phase: cytokinesis (or cell division), in which a cell's cytoplasm splits in half to create two different daughter cells, and mitosis, in which the cell's chromosomes are divided equally between the two daughter cells.
Mitosis and it's significance
Mitosis and its significance
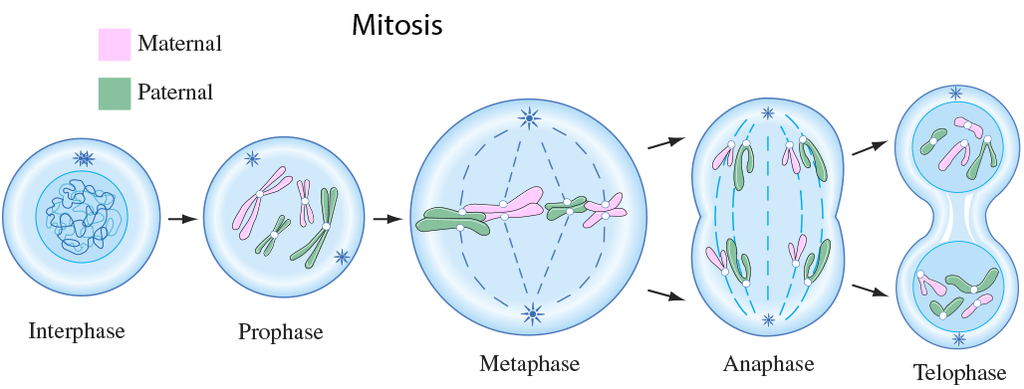
A cell prepares for cell division by replicating its chromosomes, segregating them, and creating two identical nuclei during the mitotic phase. The cell's contents are often evenly divided into two daughter cells with identical genomes after mitosis.Mitosis is divided into the following four stages:
1. Prophase:
Interphase's S and G2 phases are followed by prophase, the initial step of mitosis. The newly synthesised DNA molecules are not distinct but rather entangled in the S and G2 phases. The beginning of chromosomal material condensing characterises prophase. During the process of chromatin condensation, the chromosomal material is untangled. The centriole, which underwent duplication during interphase's S phase, now starts to migrate in the direction of the cell's opposing poles.Thus, the following distinctive occurrences can indicate the end of prophase:
- Compact mitotic chromosomes are created when chromosomal material condenses.Two chromatids are observed to be joined together at the centromere to form chromosomes.
- The beginning of the mitotic spindle's construction, the microtubules, and the proteinaceous elements of the cell cytoplasm aid in the process.
- The golgi complex, endoplasmic reticulum, nucleolus, and nuclear envelope are absent from cells near the end of prophase when they are observed under a microscope.
2. Metaphase:
The second phase of mitosis begins when the nuclear envelope completely disintegrates, and as a result, the chromosomes are dispersed throughout the cell's cytoplasm. The chromosome condensation process is now complete, and the chromosomes may be seen clearly under a microscope. Therefore, this is the period at which it is easiest to study the shape of chromosomes. The two sister chromatids that make up the metaphase chromosome at this point are joined by the centromere. Kinetochore refers to a little disc-shaped structure at the centromere surface. These structures act as the points of attachment for the spindle fibres, which are created by the spindle fibres, to the chromosomes that are placed at the cell's centre.Thus, all of the chromosomes align at the equator during the metaphase, with one chromatid of each chromosome attached by its kinetochore to spindle fibres from one pole and its sister chromatid connected by its kinetochore to spindle fibres from the opposite pole. The term "metaphase plate" refers to the chromosomes' alignment plane during metaphase. Metaphase's primary characteristics are:
- Spindle fibres adhere to chromosomal kinetochores.
- Chromosomes are transferred to the spindle equator, where they are positioned along the metaphase plate and along the spindle fibres to both poles.
3. Anaphase:
Each chromosome on the metaphase plate splits simultaneously at the start of anaphase, and the two daughter chromatids, which are now known as the chromosomes of the future daughter nuclei, start to move in opposite directions. The centromere of each chromosome is at the pole and, as a result, at the leading edge, with the arms of the chromosome trailing behind as they advance away from the equatorial plate. Events that define the anaphase stage are:
- Centromeres divide, and chromatids dissociate.
- Chromatids shift to the polar opposites
4. Telophase:
The chromosomes that have reached their respective poles decondense and lose their identity at the beginning of the last stage of mitosis, known as telophase. The individual chromosomes are no longer visible, and the two poles tend to accumulate a mass of chromatin material. The essential events at this stage include:
- Chromosomes cluster at opposing spindle poles, losing their identity as distinct elements.
- The nucleolus, Golgi complex, and ER remodel themselves
- The nuclear envelope forms around the chromosomal clusters.
Cytokinesis:
In addition to segregating duplicated chromosomes into daughter nuclei (karyokinesis), mitosis also divides the cell into two daughter cells by a separate process known as cytokinesis, marking the completion of cell division. This occurs when a furrow forms in the plasma membrane of an animal cell. The cytoplasm of the cell is split in half by the furrow, which eventually merges in the centre. Plant cells, on the other hand, are surrounded by a cell wall that is rather inextensible; as a result, they go through cytokinesis using a different process. In plant cells, wall construction begins in the cell's middle and extends outward to meet the lateral walls that already exist.The basic precursor known as the cell-plate, which symbolises the middle lamella between the walls of two neighbouring cells, is formed before the new cell wall can be fully formed. Organelles like mitochondria and plastids are dispersed between the two daughter cells during cytoplasmic division. In some organisms, cytokinesis does not follow karyokinesis, which results in a multinucleate state and the development of syncytium.
Significance of Mitosis :
Only diploid cells often undergo mitosis, also known as equational division. However, haploid cells can also divide through mitosis in some lower plants and social insects. Understanding the importance of this division in an organism's life is crucial. Typically, mitosis produces daughter cells that are diploid and have the same genetic makeup. Mitosis is responsible for multicellular organisms' growth. The ratio of the nucleus to the cytoplasm is disturbed as a result of cell expansion. Therefore, cell division is required to re-establish the nucleo-cytoplasmic ratio. Cell repair is one of mitosis' most important functions.Blood cells, stomach lining cells, and the outermost layer of the epidermis all undergo continuous replacement. Plants grow continuously throughout their lives as a result of mitotic divisions in the meristematic tissues known as the apical and lateral cambium.
meiosis and it's significance
Meiosis and its significance
Meiosis is a type of cell division that results in the production of four gamete cells and a 50% reduction in the number of chromosomes in the parent cell. To develop egg and sperm cells for sexual reproduction, this process is necessary. There are four haploid daughter cells formed during meiosis (containing half as many chromosomes as the parent cell).Following are the main characteristics of meiosis:
- Meiosis requires just one cycle of DNA replication but two successive cycles of nuclear and cell division called meiosis I and meiosis II.
- After the paternal chromosomes have duplicated to form identical sister chromatids at the S phase, meiosis I begins.
- Meiosis involves the pairing and recombination of homologous chromosomes.
- At the end of meiosis II, four haploid cells are produced.
Events of Meiosis are classified as:
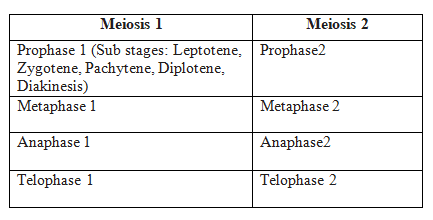
Meiosis 1
Prophase I: When compared to the prophase of mitosis, the prophase of the first meiotic division is often longer and more complex. Based on chromosomal behaviour, it has been further split into the following five phases: Leptotene, Zygotene, Pachytene, Diplotene, and Diakinesis.
Leptotene: The chromosomes begin to condense at this stage and are connected to the nuclear membrane via their telomeres.
Zygotene: A synaptonemal complex forms between homologous chromosomes at the start of synapsis.
Pachytene - During this stage, genetic material is transferred between chromatids that are not sisters. This is called crossing over.
Diplotene - At this stage, homologous pairs are still connected at the chiasmata after synapsis has ended and the synaptonemal complex has vanished.
Diakinesis: Before metaphase 1, the nuclear membrane finally breaks down and the chromosomes are entirely condensed.
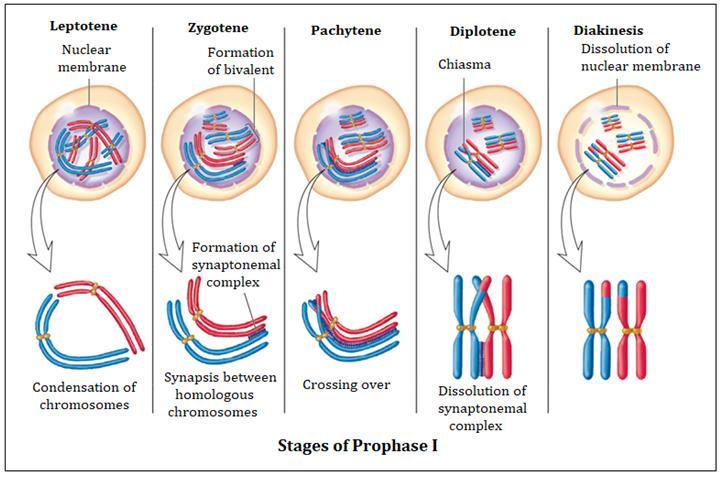
Metaphase I: The bivalent chromosomes line up on the equatorial plate during metaphase I. The spindle's opposing poles' microtubules connect to the pair of homologous chromosomes.
Anaphase I: Sister chromatids are still connected at their centromeres during anaphase I, but homologous chromosomes split.
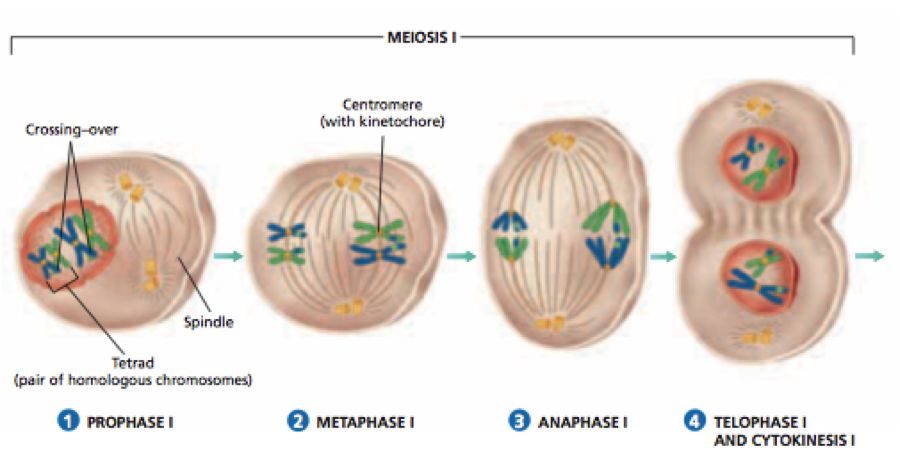
Telophase I: Cytokinesis occurs after the nuclear membrane and nucleolus re-emerge, and this is known as the "diad of cells." Even though the chromosomes do experience some dispersion in many instances, they rarely reach the interphase nucleus's very stretched state. Interkinesis, which occurs between the two meiotic divisions, is typically a transient stage. Prophase II, which is substantially less complex than prophase I, comes after interkinesis.
Meiosis 2:
Prophase II: Following cytokinesis, meiosis II begins right away, typically before the chromosomes have fully expanded. Meiosis II resembles a typical mitosis in contrast to meiosis I. By the end of prophase II, the nuclear membrane is gone. Chromosomes once more become condensed.
Metaphase II: Chromosomes align at the equator during metaphase II, and sister chromatids' kinetochores get attachments of microtubules from the spindle's opposing poles.
AnaphaseII: Beginning with the simultaneous division of each chromosome's centromere (which had been binding the sister chromatids together), anaphase II allows the chromosomes to migrate toward their respective poles of the cell.
Telophase II: Telophase II marks the completion of meiosis, during which the two chromosomal groups are once more encased in a nuclear membrane. Cytokinesis then takes place, culminating in the production of a tetrad of cells, or four haploid daughter cells.
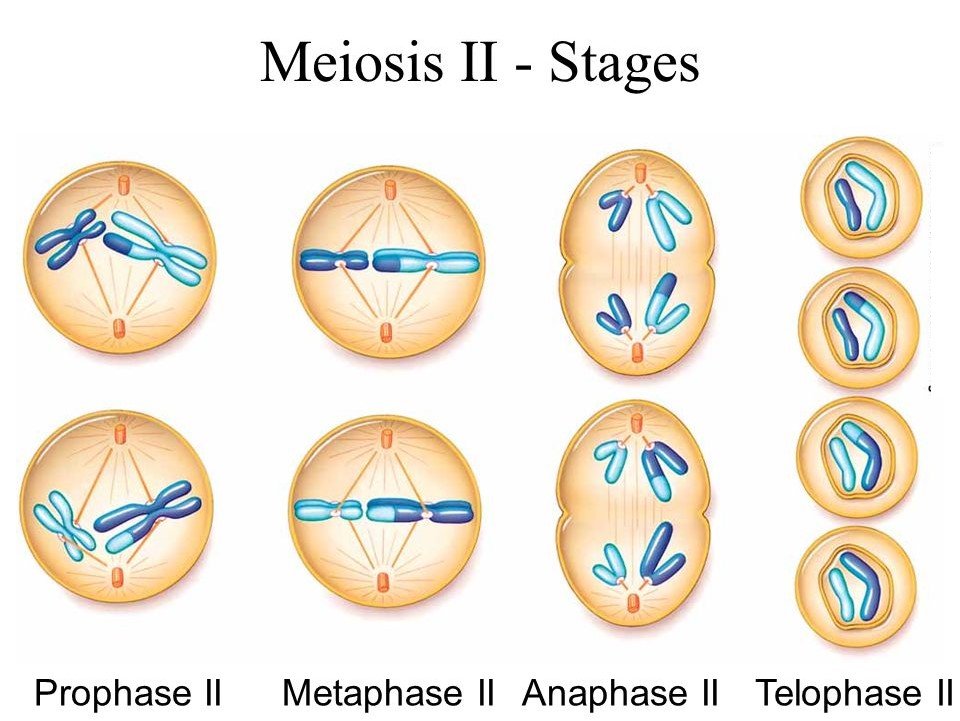
Significance of Meiosis:
Even though the process itself paradoxically reduces the number of chromosomes by half, meiosis is the mechanism that allows sexually reproducing animals to maintain the particular chromosomal number of each species throughout generations. From one generation to the next, it also makes the population of organisms more genetically variable. For the process of evolution, variations are crucial.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
