- Books Name
- ACME SMART COACHING Biology Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Biology
Plant water relation
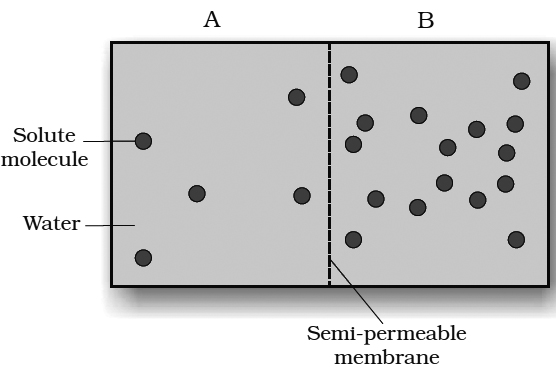
Membrane permeability
Permeability is the degree of diffusion of gases, liquids and dissolved substances through a membrane.
Normally, permeability of a given membrane to a particular substance remains unchanged.
However, changes in permeability can be brought about by many artificial and natural changes in the environment.
Following four types of membranes have been recognized on the basis of permeability:
1.Permeable. This type of membrane allows a free diffusion of both solvent and solute or ions through them. e.g., plant cell wall i.e., cellulosic and lignified.
2.Impermeable. The membranes with deposits of cutin and suberin do not allow the entry of water, dissolved substances and gases, hence, called impermeable, e.g., cell wall with thick layer of cutin on its surface.
3.Semi-permeable. A membrane that is impermeable to solute molecules but is permeable to the solvent is called a semipermeable membrane. This forms a perfect partition between two osmometers. e.g., copper ferricyanide membrane, parchment membrane, cellophane, collodion membranes.
4.Selectively or differentially permeable membrane. This membrane allows some molecules and ions to enter readily, while allowing others more slowly and does not allow certain molecules at all (i.e., different substances diffuse at different rates). Biological membranes, particularly the cell membrane (plasmalemma), tonoplast (vacuolar membrane) and the membranes surrounding sub cellular organelles are selectively permeable.
A variety of biologi.cal phenomenon are used to explain plant water relations, like osmosis, turgor pressure, wall pressure, DPD, water potential and imbibition. These are described separately in detail.
I. Osmosis (Term by Nollet)
Osmosis is a diffusion of solvent molecules through a semipermeable membrane.
Osmosis may be defined as the passage of solvent molecules from a region of their higher concentration to a region of their lower concentration through a semipermeable membrane.
Osmosis can be demonstrated by a simple experiment in laboratory.
Animal bladder or parchment membrane is tied to the wide mouth of a thistle funnel.
Concentrated sugar solution or syrup is filled in the tube of the thistle funnel.
Now, the wide mouth of thistle funnel is immersed in a beaker containing water.
The level of the solution in the tube of the funnel is marked.
After sometime, the level in the tube increases.
This is due to the entry of water molecules from beaker into the thistle funnel.
The concentration of water molecules in the beaker is more than their concentration inside the thistle funnel.
Therefore, water molecules move from the region of their higher concentration (i.e., from beaker) to the region of their lower concentration (i.e, inside the funnel).
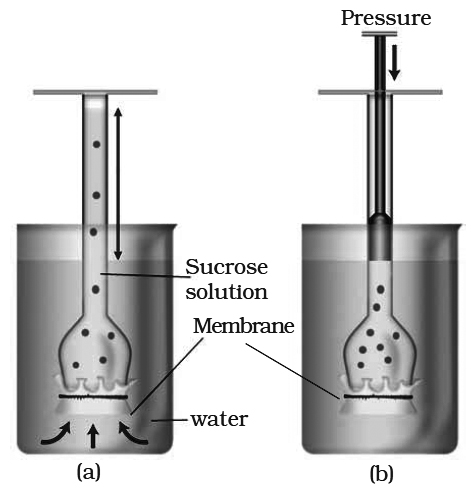
A thistle funnel is filled with sucrose solution and kept inverted in a beaker containing water. (a) Water will diffuse across the membrane (as shown by arrows) to raise the level of the solution in the funnel (b) Pressure can be applied as shown to stop the water movement into the funnel.
1.Endosmosis. When a cell is placed in a hypotonic solution water will enter into the cell from the outer (hypotonic) solution. It is because the cell sap is more concentrated (has less water molecules) than the outer solutions. This process of diffusion of water into the cell from outside is known as endosmosis. It will result in increase in the volume of the cell. e.g., Raisins placed in water.
2.Exosmosis. When a cell is immersed in hypertonic solution, water will diffuse out of the cell, because the concentration of water molecules in the cell is more than the outer solution. This process is described as exosmosis. e.g., grapes placed in sugar solution.
Osmotic Pressure (Term by Pfeffer)
It is the "maximum pressure which can develop in a osmotically active solution when it is separated from pure water by a semi-permeable membrane".
It is also defined as "the pressure needed to prevent the passage of pure water into an aqueous solution through a semipermeable membrane thereby preventing an increase in the volume of the solution".
The osmotic pressure (OP) depends upon (i) the concentration of solute particles, (ii) ionisation of solute particles, (iii) hydration of solute particles, (iv) temperature.
An increase in the condmtration of solutes in the solution increases the osmotic pressure.
If the solute ionises in solution, the number of particles increase, thus raising the osmotic pressure.
If solute molecules are hydrated, the water molecules bound with the solute are not able to diffuse and hence increase the osmotic pressure.
An increase in temperature raises the osmotic pressure of solution.
Plant cells exhibit a considerable range of variations in osmotic pressure.
In land plants, it varies from 5-30 atms. In aquatic plants, it varies from 1-3 atmp.
Plants of arid regions possess high OP.
Highest osmotic pressure is recorded in a halophytic plant, Atriplex confertifolia i.e., 202.5 atms.
OP of electrolyte is 2-3 times greater than a nonelectrolyte.
Osmotic pressure can be calculated by
OP(π ) = miRT [m =Molar concentration, i = Ionization constant, R = Gas constant,
T =Temperature (273°K)]
Osmotic pressure is numerically equal to osmotic / solute potential (Ψs) but has a positive value
Ψs = -π
Factor affecting OP
(1) Concentration of solute particles: OP of solution depends upon the concentration of solute in a given solvent. If the concentration of solute is increased then the OP of solution is also increased.
(2) Ionization of the solute molecule: OP of a solution depends upon the ionization of solute. Increased number of ions increases the OP.
(3) Temperature: OP of solution increases with increase in temperature.
(4) Hydration: Hydrated solute molecules will decrease the number of free water molecules, thereby increasing OP.
Reverse Osmosis:
It is the reverse movement of water through a semipermable membrane from a more concentrated solution to a more dilute solution by applying external pressure on the more concentrated solution, the pressure required to bring about reverse osmosis is more than the one required to prevent osmotic entry of water into it.
The method pushes out pure water.
Reverse osmosis is used in chemical industries to obtain pure water and also to purify water for drinking purposes.
Role of osmosis
1.Plants absorb water by osmotic mechanism.
2.Movement and distribution of water across the cells occur by osmosis.
3.Opening and closing of stomata is affected by osmosis.
4.The resistance of plants to drought and frost increases with increase in osmotic pressure of their cells.
5.Growth of young cells is due to osmotic pressure and turgor pressure.
6.Maintenance of turgidity.
Types of Solutions
In relation to cell sap, solutions can be of following three types:
1.Hypertonic solution. A solution whose concentration is more than that of the cell sap is known as hypertonic. If a cell is placed in such a solution, water will diffuse out of it (exosmosis) and the protoplasm would contract or shrink.
2.Hypotonic solution. When the concentration of a solution is less than that of the cell sap, it is known as hypotonic. If a cell is immersed in hypotonic solution, water will diffuse into the cell (endosmosis) and it will increase in size.
3.Isotonic solution. A solution with concentration equal to that of cell sap, is known as isotonic. If a cell is placed in isotonic solution there would be no net diffusion of water. As a result there is no change in the volume and weight of the cell.(Neither endosmosis nor exosmosis).
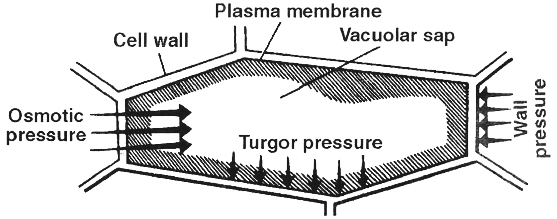
II. Turgor Pressure (TP)
It is a pressure which is developed in an osmotic system due to entry of water.
It causes swelling of the system.
Protoplasm of a plant cell functions as an osmotic system such that on absorption of water it becomes turgid and the turgid protoplast presses the cell wall towards the outside with a force called turgor pressure.
1. The cell wall also exerts a pressure over the protoplast, it is called wall pressure (WP).
2. Normally wall pressure is equal but opposite to turgor pressure (WP = TP).
3. Turgor pressure and wall pressure are positive pressures (negative TP is characteristic of plasmolysed cell and xylem vessels).
4. Reduced turgor pressure results in loss of turgidity.
5. Turgor change is also responsible for different types of plant movements and also for stomatal movement.
III. Diffusion Pressure Deficit (DPD) (term by Meyer) or
Suction Pressure (SP) (term by Renner)
Each liquid has a specific diffusion pressure.
Pure water has the maximum diffusion pressure.
The solution prepared by dissolving solute (such as sugar or salt) in pure water has lesser diffusion pressure as compared to pure solvent or water (though the solution has higher osmotic pressure).
In this way, there is always a difference between the diffusion pressure of solvent and its solution. Therefore, diffusion pressure deficit (DPD) may be defined as the difference between the diffusion pressure of a solution and a pure solvent, when both are subjected to the same atmospheric pressure.
To remove this deficit, the solution would absorb more solvent molecules, means water moves from low DPD to high DPD.
In this way, diffusion pressure deficit is the water absorption capacity of a solution.
Therefore, DPD can also be called as suction pressure. Its value is always positive for a cell.
Diffusion Pressure Deficit = Osmotic pressure - Turgor pressure
i.e., DPD = OP - TP
The above relationship indicates, that
1.When a cell is flaccid, its DPD = OP.
2.The entry of water into the cell causes development of TP.
3.When the increasing turgor pressure becomes equal to that of decreasing osmotic pressure, the entry of water into the cell would stop. This is called turgid condition of the cell. This can be expressed as
OP - TP = 0, thus DPD = 0
Concept of Water Potential
Water moves from region of higher free energy to that of lower free energy.
Thus, the net movement of water molecules occurc down the energy gradient.
The free energy of solvent can be increased by increasing the temperature and pressure.
The difference between the free energy of water molecules in pure water and the free energy of water in any other system (e.g., water in the solution or in a cell) is termed the water potential of that system.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
