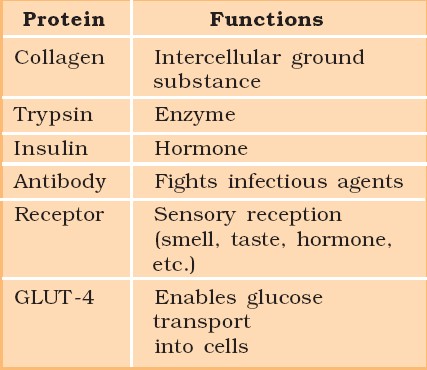
Proteins
Polypeptides are the building blocks of proteins. They are peptide bonds that connect linear chains of amino acids. A polymer of amino acids makes up each protein. A protein is a heteropolymer, not a homopolymer because there are 20 different types of amino acids. A homopolymer is made up of only one type of monomer that repeats 'n' times. Specific amino acids are necessary for human health and must be obtained through our food. As a result, necessary amino acids are obtained from food proteins. As a result, amino acids can be classified as either essential or non-essential. The latter are those that our bodies can produce, whereas essential amino acids must be obtained through our diet.Proteins have a variety of activities in living creatures, including transporting nutrients across cell membranes, fighting pathogenic organisms, acting as hormones, and enzymatic reactions. The most abundant protein in the animal kingdom is collagen, and the most abundant protein in the biosphere is Ribulose bisphosphate Carboxylase-Oxygenase (RuBisCO).
Structure of Proteins:
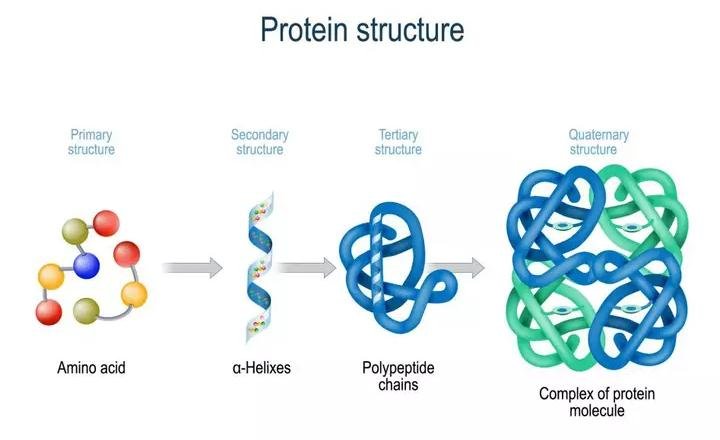
Proteins are heteropolymers made up of strings of amino acids, as previously stated. In different situations, the structure of molecules means different things. Physicists visualize molecular structures in three dimensions, while biologists explain protein structure on four levels. The primary structure of a protein is the sequence of amino acids, or the positional information in a protein — which amino acid is the first, which is the second, and so on.A protein can be visualized as a line, with the initial amino acid on the left end and the last amino acid on the right.
N-terminal amino acid is another name for the first amino acid. The C-terminal amino acid is the last one in the chain. As an extended stiff rod, a protein thread does not exist throughout. The thread is folded into a helix shape (similar to a revolving staircase). Naturally, only a piece of the protein thread is organized in a helix shape. Only right-handed helices are found in proteins. In what is known as the secondary structure, various portions of the protein thread are folded into other configurations.
Furthermore, the lengthy protein chain folds in on itself like a hollow woollen ball, forming the tertiary structure. This allows us to see a protein in three dimensions. Proteins' tertiary structure is required for many of their biological functions. Some proteins are made up of several polypeptides and subunits. The architecture of a protein, also known as the quaternary structure of a protein, is the way these individual folded polypeptides or subunits are arranged with regard to one other (e.g., a linear string of spheres, spheres placed one upon another in the form of a cube or plate, etc.).Adult human haemoglobin consists of 4 subunits. Two of these are identical to each other. Hence, two subunits of α type and two subunits of β type together constitute the human haemoglobin (Hb).
Nature of bond linking monomers in a polymer
A peptide bond is produced when the carboxyl (-COOH) group of one amino acid combines with the amino (-NH2) group of the next amino acid with the elimination of a water molecule in a polypeptide or protein (the process is called dehydration). Individual monosaccharides in a polysaccharide are joined by a glycosidic bond. Dehydration also contributes to the formation of this link. Two carbon atoms from two neighboring monosaccharides make a peptide bond. A phosphate moiety of a nucleic acid connects the 3'-carbon of one sugar of one nucleotide to the 5'-carbon of the sugar of the next nucleotide. An ester link exists between the phosphate and the sugar's hydroxyl group.The phosphodiester bond is named after the fact that there is one such ester bond on each side.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
