Matter in Our Surroundings
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 1
Matter in Our Surroundings
Matter in Our Surroundings
What We Learn in Day 1
- Matter in Our Surroundings
- Matter is made of Particles
- Evidence for particles in Matter
The process of dissolving sugar in water gives us two conclusions about the nature of matter
The existence of Brownian motion gives us two conclusions about the nature of matter
Characteristics of Particles of Matter
Matter in Our Surroundings Anything which occupies space and has mass is called matter.

Matter is made of Particles
The particles which make up matter are atoms or molecules.
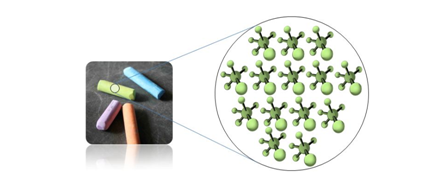
Evidence for particles in Matter
This movement of different particles among each other (on their own), so that they become mixed uniformly, is called diffusion. When sugar is added to water and stirred, it dissolves quickly.
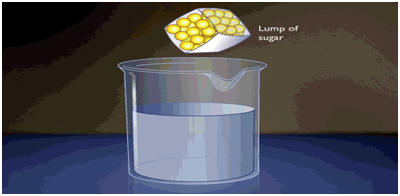
When sugar dissolves sugar particles go into the spaces between the particles of water

When KMno4 dissolves KMno4 particles go into the spaces between the particles of water
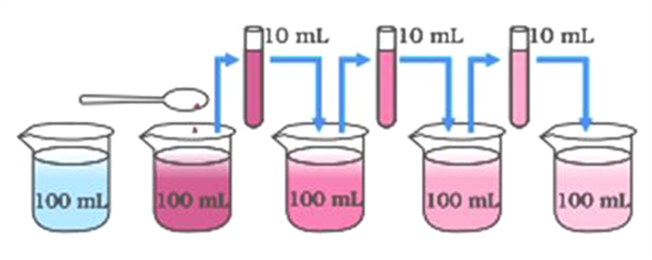
The process of dissolving sugar in water gives us two conclusions about the nature of matter
- That matter is made up of tiny particles, and
- That the particles of matter are constantly moving.
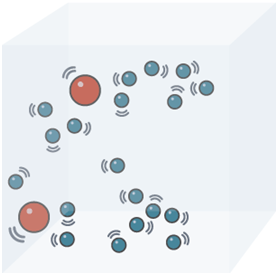
The existence of Brownian motion gives us two conclusions about the nature of matter
- That matter is made up of tiny particles, and
- That the particles of matter are constantly moving.
The zig – zag movement of the small particles suspended in a liquid (or gas) is called Brownian motion. Brownian motion increases on increasing the temperature.
Characteristics of Particles of Matter
- They are very small.
- They have spaces between them.
- They are constantly moving.
- They attract each other
Classification of Matter
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Classification of Matter
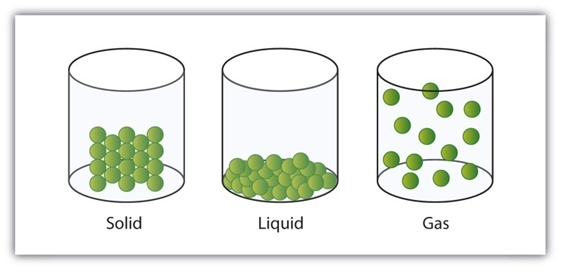
- On the basis of its physical state, such as solid, liquid or gas.
- On the basis of its chemical composition, such as an element compound or mixture.
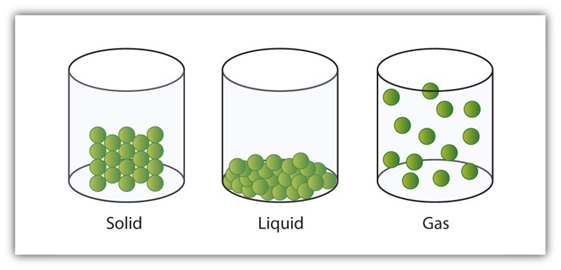
Matter
- Solid
- Liquid
- Gas
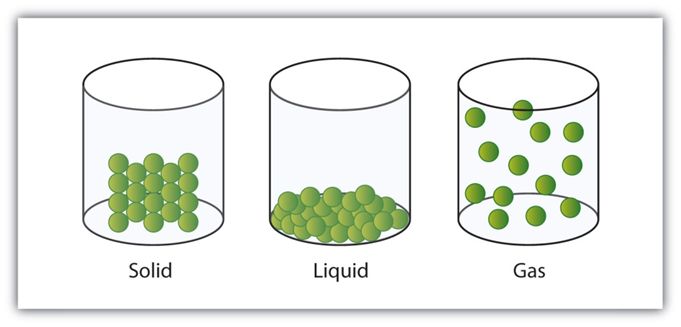
Properties of Solids
- Solids have a fixed shapes and a fixed volume.
- Solids cannot be compressed much.
- Solids have high densities. They are heavy.
- Solids do not fill their container completely.
- Solids do not flow.
Properties of Liquids
- Liquids have a fixed volume but they have no fixed shape.
- Like solids, liquids cannot be compressed much.
- Liquids have moderate to high densities.
- Liquids can take the shape of container in which they kept..
- Liquids generally flow easily.

Properties of Gasses
- Gases have neither a fixed shape nor a fixed volume.
- Gases can be compressed easily
- Gases have very low densities.
- Gases fill their container completely.
- Gases flow easily.

Why Solids, Liquids and Gases Have Different Properties
According to the kinetic theory of matter, the particles of matter are in continuous motion.
Some force of attraction tends to hold the particles together and control their movements.
To Show that Liquids do not have a Fixed Shape but they have a Fixed Volume
Does Gases have fixed shape and fixed volume why?
Why Solids and Liquids Cannot be Compressed but Gasses Can be Compressed Easily?
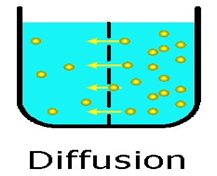
Diffusion
Diffusion is a property of matter which is based on the motion of its particles. Diffusion is fastest in gases and slowest in solids. The rate of diffusion increases on increasing the temperature.
The Common Unit of Temperature and SI Unit of Temperature
We use Celsius scale of temperature for measuring temperatures in our everyday life.
The laboratory thermometers which we use for performing science experiments the clinical thermometer which we use for measuring human body temperature
K = oC + 273
OoC = 273K
Change of state of matter 1
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Change of state of matter
Why Solids, Liquids and Gases Have Different Properties
According to the kinetic theory of matter, the particles of matter are in continuous motion.
Some force of attraction tends to hold the particles together and control their movements.
To Show that Liquids do not have a Fixed Shape but they have a Fixed Volume
Does Gases have fixed shape and fixed volume why?
Why Solids and Liquids Cannot be Compressed but Gasses Can be Compressed Easily?
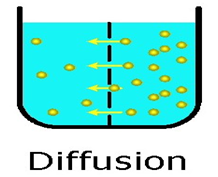
Diffusion
Diffusion is a property of matter which is based on the motion of its particles. Diffusion is fastest in gases and slowest in solids. The rate of diffusion increases on increasing the temperature.
Illustration
When someone opens a bottle of perfume in one corner of a room, its smell spreads in the whole room quickly. Why?
Solution
This happens because the particles of perfume (GAS) move rapidly in all the directions and mix with the moving particles of air in the room.
Illustration
Give reasons for the following observation. The smell of hot sizzling food reaches you several meters away, but to get the smell of cold food you have to go close.
Solution
The vapours of hot food diffuse in air readily but the cold food does not evaporate so fast so its particles cannot diffuse quickly in air and its smell cannot travel long distance.
The Common Unit of Temperature and SI Unit of Temperature
We use Celsius scale of temperature for measuring temperatures in our everyday life.
The laboratory thermometers which we use for performing science experiments the clinical thermometer which we use for measuring human body temperature
K = oC + 273
OoC = 273K
Illustration
Convert the following temperature to Celsius scale
- 300K
- 573K
Solution
For converting Kelvin to Celsius, the formula is
K – 273 = oC
- 300K – 273 = 27oC
- 573K – 273 = 300oC
Illustration
Convert the following temperature to Celsius scale
- 293K
- 470K
Solution
For converting Kelvin to Celsius, the formula is
K – 273 = oC
- 293K – 273 = 20oC
- 470K – 273 = 197oC
Illustration
Convert the following temperature to Kelvin scale
- 25oC
- 373oC
Solution
For converting Celsius to Kelvin, the formula is
oC + 273 = K
- 25oC + 273 = 298K
- 373oC + 273 = 646K
Change of state of matter
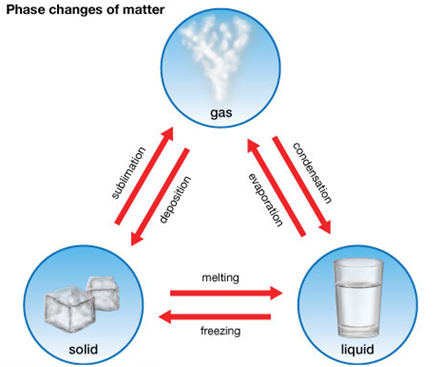
We can change the physical state of matter in two ways:
- By changing the temperature, and
- By changing the pressure.
Solid to Liquid Change: Melting
The process, in which a solid substance changes into a liquid on heating, is called melting (or fusion).
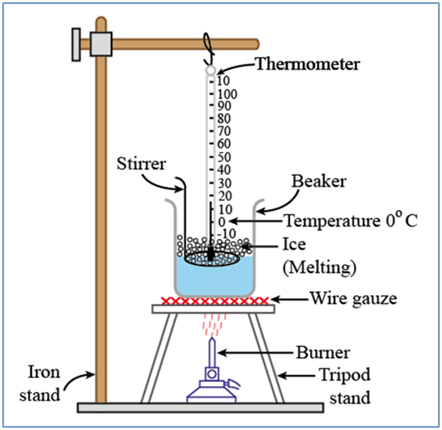
Illustration
For any substance, why does the temperature remain constant during the change of state?
Solution
During the change of state, the temperature becomes constant, because the heat provided is utilized for breaking the attraction force between the particles of the substance. This happens at melting point (or boiling point) of the substance and the heat used is called the latent heat of fusion (or vaporization). During condensation or solidification, the vice-versa happens.
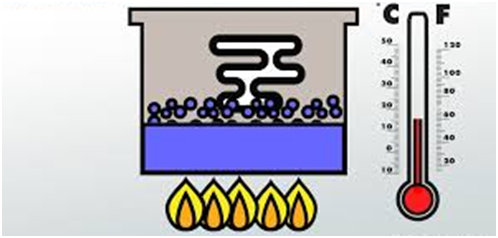
Liquid to Gas Change: Boiling (or Vaporization)
Place during boiling. The boiling of a liquid takes place at a fixed temperature. The temperature, at which a liquid boils and changes rapidly into a gas at atmospheric pressure, is called boiling point of the liquid.
When a liquid is heating, it changes its physical state and becomes a gas.
Illustration
What would be the temperature when water starts boiling?
Solution
As the water starts boiling, the temperature will be 100oC or 373 K.
Illustration
What is the temperature when all the water has boiled?
Solution
The temperature remains constant i.e., 100oC during its complete boiling process.
Illustration
Under which condition, we can boil water at room temperature?
Solution
We can boil water at room temperature 1 atomic pressure.
Illustration
At what temperature boiling takes place?
Solution
Boiling takes place only at boiling point.
Gas to Liquids Change:- Condensation
The process of changing a gas to liquid by cooling is called condensation. 

Liquid to Solid Change: Freezing
The process of changing a liquid into a solid by cooling is called freezing.
When a liquid is cooled its particles lose energy due to which they move slowly. At this stage the liquid freezes and becomes a solid.
Illustration
Give reason for the observations. We can get the smell of perfume sitting several meters away?
Solution
The smell o aroma of perfume reaches several meters away due to the fast diffusion of the gaseous particles of perfume through air.
Latent Heat
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Latent Heat
The heat energy which has to be supplied to change the state of a substance is called its latent heat. Latent heat does not raise (or increase) the temperature.
Latent Heat is of Two Types
- Fusion
- Vaporization
Latent Heat of fusion (Solid to Liquid Change)
The latent heat of fusion (or melting) of a solid is the quantity of heat in joules required to convent 1 kilogram of the solid (at its melting point) to liquid, without any change in temperature.
Latent Heat of Vaporization
It is defined as heat required converting 1 mole of liquid into vapors completely at its boiling point.
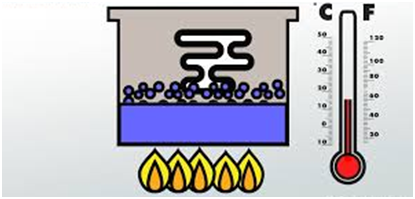
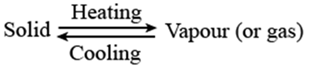
Sublimation
The changing of a solid directly in to vapors on heating. And vapors’ into solid on cooling is known as sublimation. Sublimation can be represented as:
Ex:- Solids carbon dioxide
is a white solid called dry ice.
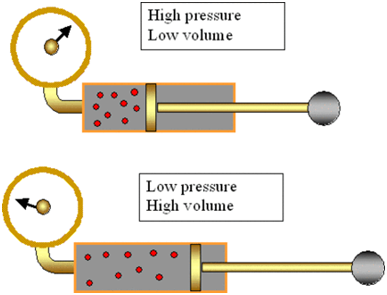
Gases Can be Liquefied by Applying Pressure and Lowering Temperature
Gases can be liquefied by applying pressure and lowering temperature.
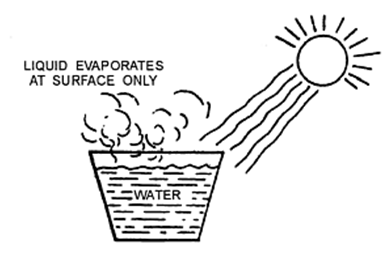
Evaporation
The process of a liquid changing into vapour (or gas) even below its boiling point is called evaporation.
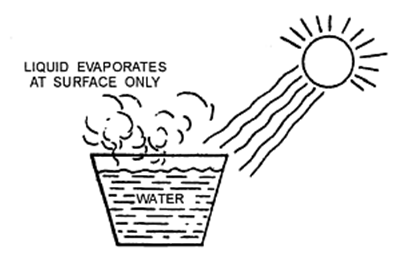
Factors Affecting Evaporation
The evaporation of a liquid depends mainly on the following factors:
- Temperature
- Surface area
- Humidity
- Wind speed
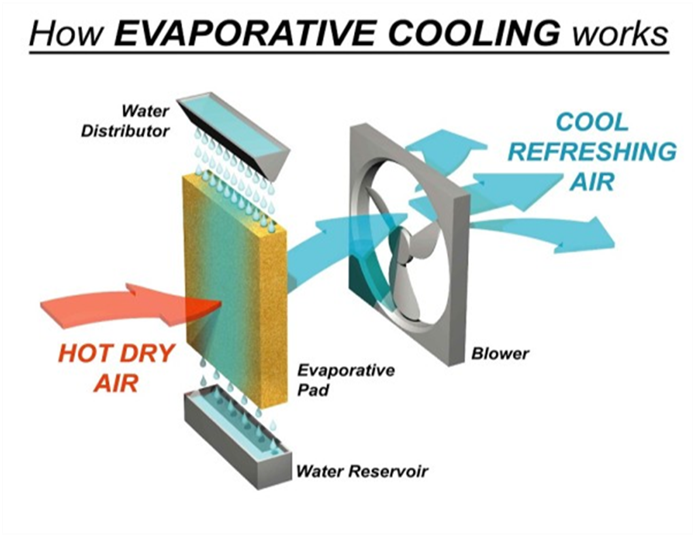
Cooling Caused by Evaporation
The cooling caused by evaporation is based on the fact that when a liquid evaporates, it draws the latent heat of vaporization from ‘anything’ which it touches and makes it cool.
Matter (Solid, Liquid or Gas)
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 2
Is Matter around us Pure
Matter (Solid, Liquid or Gas)
- Pure Substance,
- Mixtures (No Fixed Composition)
Pure Substance
- Elements (Cannot be broken down to simpler substances)
Examples:-
Copper, oxygen, iron, hydrogen, mercury etc.
- Compounds (Have fixed composition can be broken down into elements by chemical or electrochemical reactions)
Examples:-
Water, methane, sugar, salt etc.
Mixtures (No Fixed Composition)
- HomogeneousUniform composition
- HeterogeneousNon-Uniform composition
Homogeneous (Uniform composition)
Examples:-
Sugar in water, salt in water, sulphur in carbon
disulphide, water in alcohol etc.
Heterogeneous Non-Uniform composition
Examples:-
Sand and salt, sugar, and salt water in oil etc.
Mixtures
When two or more substances (elements/ compounds) are mixed together in any ratio, but they do not combine chemically, a mixture is formed.
Types of Mixtures
Depending upon the nature of the components that form a mixture, we have two types of mixtures.
Mixtures (No Fixed Composition)
- Homogeneous
- Heterogeneous
Homogeneous Mixtures
Air, alloy, soda water etc.

Heterogeneous Mixtures
A mixture of oil and water suspensions colloids etc

Components of a Solution
- Solvent
- Solute
Solvent
The component of the solution that dissolves the other component in it (usually the component present in larger amount) is called the solvent.
Solute
The component of the solution that is dissolved in the solvent (usually present in lesser quantity) is called the solute.
Types of Solution
Depending upon the amount of solute present in a given amount of solvent, the
solution can be classified into following three classes
Types of Solution
- Saturated Solution
- Unsaturated Solution
- Supersaturated solution
Saturated Solution
A solution in which no more amount of solute can be dissolved at a given temperature is known as saturated solution. The amount of solute present in the saturated solution at this temperature is called its solubility.
![]()

Unsaturated Solution
Some more amount of solute can be dissolved at a given temperature, is called unsaturated solution.
Supersaturated Solution If solution contains more amount of solute than the saturation concentration, it is called supersaturated solution.

Properties of a Solution
- A solution is a homogeneous mixture.
- The particles of a solution are smaller than 1 nm (10-9m) in diameter. therefore, they cannot be seen by naked eyes.
- Due to very small particles size, they do not scatter a beam of light passing through the solution. So, the path of light is not visible in a solution.
- A solution is stable. The solute particles cannot be separated by the process of filtration, also they do not settle down when left undisturbed.
Concentration of a Solution
The amount of solute present in a given amount of solution.

Suspension and Evaporation
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Suspension and Evaporation
Suspension
A suspension is a heterogeneous mixture in which the solute particles do not dissolve but remain suspended throughout the bulk of the medium.
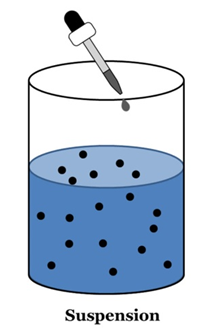
Properties of a Suspension
- Suspension is a heterogeneous mixture.
- Its particles can be seen by naked eyes.
- Its particles scatter a beam of light passing and make its path visible (Tyndall effect).
- It is unstable. The soluble particles settle suspension is left undisturbed or they can by the process of filtration.
Colloidal Solution

A colloid (or colloidal solution) is a mixture that is heterogeneous but appears to be homogeneous as the articles are uniformly spread
throughout the solution. e.g., milk, shaving cream, cheese etc.
Properties of a Colloid
- A colloid appears to be homogeneous but actually it is heterogeneous.
- A colloid appears to be homogeneous but actually it is heterogeneous.
- The size of particles of colloid is very small. They can not be seen even with a microscope.
- Its particles can pass through filter paper, therefore, a colloid cannot be separated by filtration. However, they get separated by a special technique, called centrifugation.
Some Common Examples of Colloids
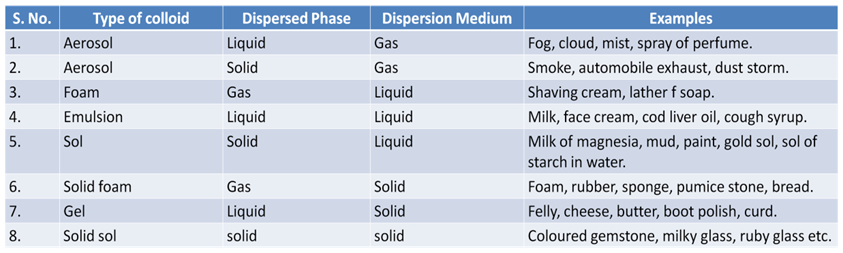
Components of a Colloid
The solute like component form the dispersed phase and the component in which the dispersed phase is suspended is known as the dispersion medium.
Evaporation
This method can be used to separate a volatile component (solvent) from a non-volatile component of a mixture.
Centrifugation
(Separation of Cream from Milk)
Two components having difference in densities can be separated by centrifugation.
Illustration
How the separation of cream from milk takes place?
Solution
To separate cream from milk, milk is churned for 2-3 minutes. Cream collects at the centre and being lighter than milk floats at the top of the mixture.
Decantation
This method is also applied where a difference between densities of components lies. A less soluble solid from a liquid can be separated by first keeping the mixture undisturbed and then decanting the liquid slowly leaving the solid in the first container.
Separating Funnel
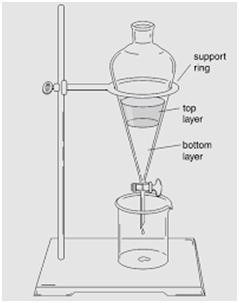
(Separation of Mixture of Two Immiscible Liquids)
This method is used for separating two immiscible liquids and is also based on the difference in densities.
Illustration
Name the apparatus used to separate these two liquids.
Solution
Separating funnel in used to separate oil from water.
Illustration
State the principle involved in this process.
Solution
This process is based upon the principle that immiscible liquids are separated out in layers depending upon their densities.
Illustration
How the layer is formed in separating funnel?
Solution
Kerosene oil being lighter will form the upper layer while water being heavier will form the lower layer.
Illustration
A mixture containing two liquids is placed in separating funnel. Answer the following.
- What type of liquids form the mixture?
- Which of the liquids will form the lower layer?
- What is the basis of this method?
Solution
- Two liquids which are immiscible with each other form the mixture.
- The heavier liquid will form the lower layer.
- The method is based on
(a):- mutual immiscibility of the two liquids
(b):- difference in the densities of the two liquids.
Sublimation and Distillation
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Sublimation and Distillation
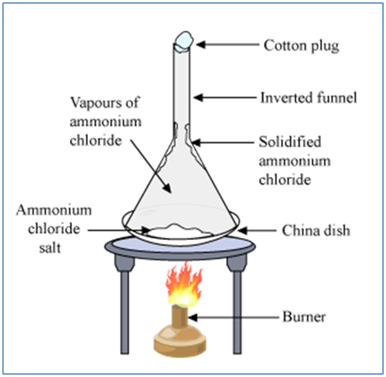
Sublimation
(Separation of a Mixture of Salt and Ammonium Chloride)
Some solids have a tendency to sublime on heating, i.e., they convert directly from solid to gaseous/vapour phase on heating through the liquid phase.
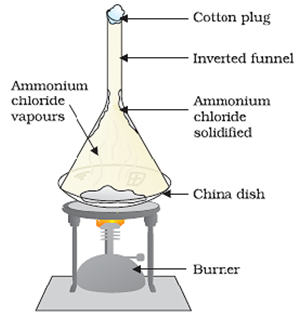
Chromatography
(Separation of Two Colour of a Dye)
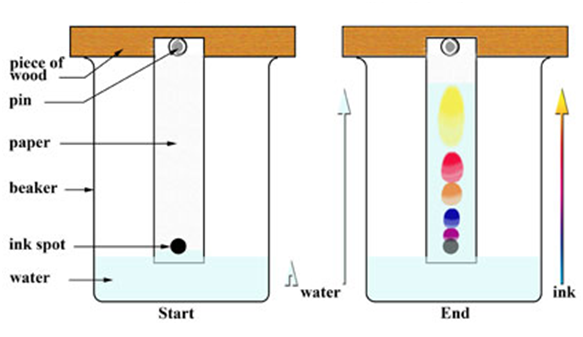
This method is used to separate a mixture of different dyes. The coloured component that is more soluble in water, rises faster and in thisway the colours get separated. This technique is generally used for the separation of those solutes that dissolve in the some solvent.
Distillation
(Separation of two Miscible Liquids)
Two miscible liquids that boil without decomposition and have sufficient difference in their boiling point (> 25° C) can la separated by simple distillation. Fractional distillation is used to separate a mixture of miscible liquids the difference it the boiling point of whichis less than 25° C.
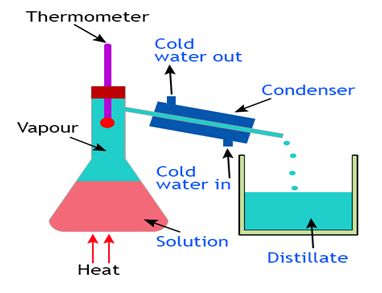
Fractional Distillation
More than components can be separated. Difference in boiling points of components may be less than 25oC. A long fractionating column is also used (where evaporation and condensation take place side by side) along with distillation flask distillation flask, condenser and receiver.
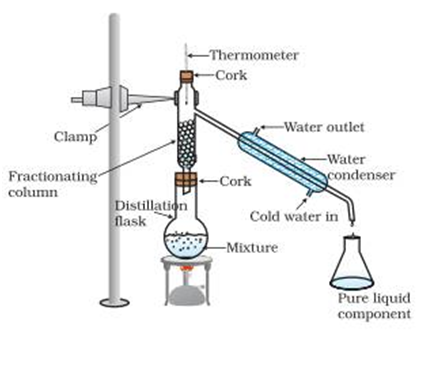
Separation of Different Gases Present in Air
Air is a homogeneous mixture of a number of gases. These can be separated from air by fractional distillation.
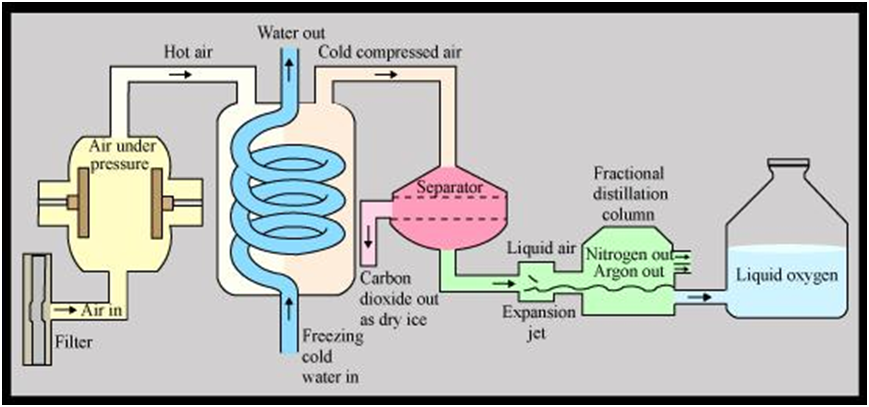
Flow diagram showing the separation of gases of air
Air (Free from CO2 and water vapours) High pressure and low (temperature)
- Liquefied Air (BP = – 200oC)Fractional distillation (Slow warming in fractionating column)
- Nitrogen Separates firs(BP = – 196oC)
- Oxygen (BP = – 183oC) Separation of constituents of air
Crystallisation
(Separation of Pure Substance from its impure Form)
Crystals are the purest form of a substance and have definite geometrical shapes. The process by which an impure compound is converted into its crystals is known as crystallisation.
Advantages of Crystallisation over Evaporation
Crystallisation is a better technique than evaporation to purify a solid due to the following reasons.
- During evaporation, the solution is heated to dryness. Some solids may decompose or some substances like sugar may get charred during direct heating.
- Some impurities may still be present in the solution after filtration and they make the solid impure.
- Direct heating or evaporation does not give crystals. Only a solid residue is left in the dish.
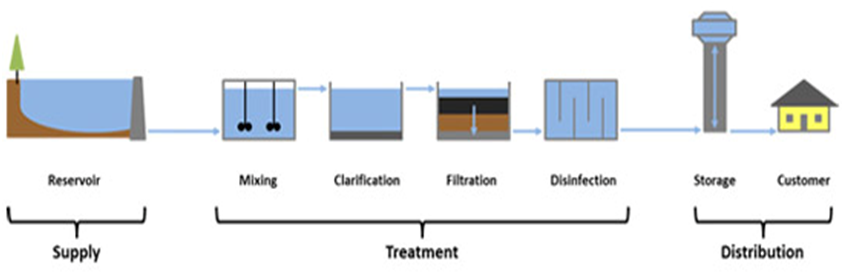
Purification of Drinking Water
In cities, drinking water is supplied from water works. The various processes used in water works for purification are:-
- Sedimentation to remove suspended solids.
- Loading with alum to remove negatively charged colliodal clay particles.
- Filtration to remove dissolved solids.
- Chlorination to kill bacteria.
Purification of Drinking Water
Pure Substance
A substance that consists of only a single type of constituent particles is called pure substance. e.g., gold, water etc.
Element
An element consists of only one type of atoms. e.g., gold, silver, hydrogen, oxygen etc. An element is a pure substance.
Elements are further classified into three groups: metals, non-metals and metalloids.
Metals and Non metals
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Metals and Non metals
Metals (Properties)
- These are lustrous.
- These are silver-gray or golden yellow in colour.
- Metals are malleable and ductile.
- Metals are good conductors of heat and electricity.
- Metals are sonorous, i.e., produce a ringing sound when hit.
Non-Metals (Properties)
- These are non-lustrous.
- The non-metals display a variety of colour.
- Non-metals are neither malleable nor ductile, but they are brittle.
- Non-metals are poor conductors of heat and electricity.
- Non-metals are non-sonorous.
Metalloids
Elements having intermediate properties between those of metals and non-metals are called metalloids. e.g., boron, silicon, germanium etc.
Compounds
A compound is a substance composed of two or more elements, chemically combined with one another in a fixed proportion. e.g., water, methane etc.
Introduction
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 3
Atoms and Molecules
Introduction
We shall discuss about the various laws which indicates how atoms combine to form molecule, symbols and formula of atoms and compounds and various ways of expressing their masses.
Laws of Chemical Combination
Whenever reactants react together to form the products or the elements combine together to form a compound, they do this according to certain laws. These laws are called the laws of chemical combination.
Law of Conservation of Mass
It states, "Mass can neither be created nor be destroyed in a chemical reaction."
Law of Constant Proportions/ Law of Definite Proportions
French chemist, joseph Proust analysed the chemical composition of a large number of compounds and came to the conclusion that the proportion of each element in a compound I constant. On this basis he proposed the law of constant proportions.
"A pure chemical compound always consists of the same elements that are combined together in a fixed (or definite) proportion by mass."
For Example:- carbon dioxide (CO2) always contains carbon and oxygen in the ratio of 3 : 8. If a sample of CO , contains 36 g of carbon then it is compulsory that it has 96g Oxygen.
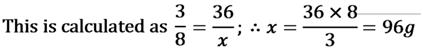
Dalton's Atomic Theory 1
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Dalton's Atomic Theory
Dalton's Atomic Theory
Dalton's atomic theory provided an explanation for the law of conservation of mass and the law of definite proportions. According to Dalton’s atomic theory, all matter (whether an element, a compound or a mixture), is compounds of small particles, called atoms.
The Main Postulates of Dalton's Atomic Theory are
- Every matter is made up of very small particles, called the atoms.
- Atoms are indivisible particles which can neither be created nor be destroyed in a chemical reaction.
- Atoms of a given element are identical in mass as well as in chemical properties.
- Atoms of different elements have different masses and chemical properties.
- Atoms combine in the ratio of small whole numbers to form compounds.
- The relative numbers as well as kinds of atoms are constant in a given compound.
Merits of Dalton's Atomic Theory
Dalton's atomic theory has proved to be very useful in various ways
- This theory has enabled us to explain the laws of chemical combination.
- Dalton was the first to recognize a workable distinction between the ultimate particle of an element (atom) and that of a compound (molecule).
Demerits of Dalton's Atomic Theory
- Atom is no longer considered as the smallest indivisible particle. It has been established that it is made up of electrons, protons and neutrons.
- Atoms of the same element may have slightly different masses (Isotopes).
- Atoms of different elements may have the same masses (Isobars).
- Substance made up of the same kind of atoms may have different properties.
- The ratio in which the different atoms combine to form compound may be fixed and integral but may not be simple.
Illustration
Dalton atomic theory postulated that "Atoms combine in simple whole number ratio". How has this postulate been modified?
Solution
According to the modified postulate, 'Atoms combine in whole number ratio but this ratio may not be simple." For example. in C12H22O11 (sucrose) ratio of C : H : O is 12 : 22 : 11, which is a whole number ratio but not the simple.
Illustration
Carbon and oxygen combine to form carbon dioxide gas. They do so in the same ratio 3 : 8. What mass of oxygen would be required to react completely with 6.0 g of carbon? Also calculate the mass of carbon dioxide gas so formed.
Solution
Carbon and oxygen combine as per law of constant proportions, i.e. 3 g of carbon will always combine with 8 g of oxygen or
∵ 3g carbon combines with oxygen = 8g
∴ 6g carbon will combine with oxygen = 8/3 x 6 = 16g
Total amount of carbon dioxide = 6 ± 16= 22 g
Atoms
Atoms are the smallest particles of an element which may or may not have independent existence but take part in a chemical reaction. These are the building blocks of all natter.
For Example:- atoms of hydrogen, oxygen, nitrogen, etc, are not capable in independent existence whereas atoms of helium, noon, etc are capable of independent existence.
Size of Atoms
Atoms are very small and their radius is measured in nanometres.
1/109 M =1 nm or lm = 109 nm
Radius of hydrogen atom is 0.1 nm.
Modern Symbols of Atoms of Different Elements
Although Dalton was the scientist who introduced symbols for representing elements for the first time but modern symbols for the elements were introduced by JJ Berzilius. These are defined as a short hand representation of the name of an element.
Now a days, it is the IUPAC who approves the names of the letters of the element’s name in English. The first letter of a symbol is always written in capital letter and the second letter as small letter.
e.g., chlorine – Cl, zinc – Zn and aluminium – Al.
Symbols of some elements have been taken from their names in different languages such as Latin, German, Greek, etc.
For Example:-
- Iron – Fe from Ferrum (Latin Name)
- Gold – Au from Aurum (Latin Name)
- Potassium – K from Kalium (Latin Name)
- Chlorine – Cl from Chloros (Greek Name)
- Cobalt – Co from Kobold (German Name)
- Sodium– Na from Natrium (Latin Name)
Atomic Mass
According to Dalton, each element has a characteristic atomic mass. But determining the mass of an individual atom was a relatively difficult task due to its very small size.
Hence, their relative atomic masses were determined using the laws of chemical combination and the compounds formed. For this purpose, initially oxygen was taken as standard because of the following two reasons:
(i):- Oxygen reacted with a large number of elements and formed compounds.
(ii):- This unit gave masses of most of the elements as whole numbers.
Relative Atomic Mass
It is defined as the number of times a given atom is heavier than 1/12th of mass of 1 atom of carbon-12 (C-12) or it is the average mass of the atom as compared to 1/12th the mass of one carbon-12 atom.
Atomic Mass Unit
It is defined as the mass unit equal to exactly 1/12th of the mass of one atom of C-12 isotope. Earlier, it was abbreviated as amu but according to latest recommendations of IUPAC, it is now written as `u'— unified mass.
Molecules
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Molecules
The smallest particle of a substance which is capable of independent existence is called a molecule. In general, molecule is a group of two or more atoms that are chemically bonded together. It shows all the properties of the substance. Molecules can be divided into two categories.
Molecules of Elements
The molecules of an element are constituted by the same type of atoms. Molecules of many elements are made up of one atom of that element. e.g. noble gases like argon (Ar), helium (He), etc.
The molecules of most of more than one atoms of the non-metals are made up of more than one atoms.
For Example:- A molecule of oxygen consists of two atoms of oxygen. Ozone (O3) consists of three atoms of oxygen.
Atomicity
It is defined as the number of atoms present in a molecule.
On the basis of atomicity, molecules can be classified as
(i):- Monatomic Molecules They consist of only one atom. e.g. He, Ne, Ar, Xe, Fe, Al, etc.
(ii):- Diatomic Molecules They consist of two atoms. e.g. H2,O2, N2,I2, Br2, C12, etc.
(iii):- Triatomic Molecules They consist of three atoms. e.g. O3.
(iv):- Tetraatomic Molecules They consist of four atoms. e.g. P4.
(v):- Polyatomic Molecules They consist of more than four atoms. e.g. S8.
Molecules of Compounds
Atoms of different elements join together in definite proportions forming molecules of compounds.
For Example, H2O molecule is made up of hydrogen and oxygen elements in the ratio of 1:8 by mass.
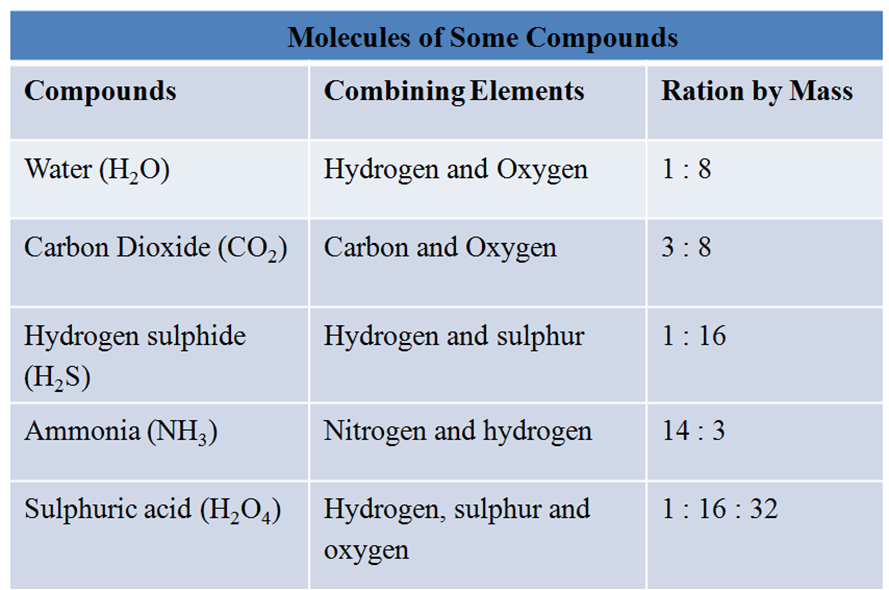
Ions
When atoms, groups of atoms or molecules lose or gain electron(s) they become charged. These charged species are known as ions. These can be negatively or positively charged.
Cations
The positively charged ions which are attracted towards cathode in an electric field are known as cations. e.g. Na+, K+, Ca2+, A13+ , etc.
Anions
The negatively charged ions which are attracted towards anode in an electric field are known as anions. e.g. Cl–, Br–, O, N3-, etc.
Ionic Compounds
The compound which consists of ions as its constituent particles is known as ionic compound. It contains ionic bonds. e.g. sodium chloride, consists of a positively charged) sodium ion (Na+ cation) and negatively charged chloride ion (C1- anion). Calcium oxide or quicklime (CaO) consist of calcium cation (Ca2+) and oxide anion (O2-).
Polyatomic Ion
The compound which consists of ions as its constituent particles is known as ionic compound. It contains ionic bonds. e.g. sodium chloride, consists of a positively charged) sodium ion (Na+ cation) and negatively charged chloride ion (C1- anion). Calcium oxide or quicklime (CaO) consist of calcium cation (Ca2+) and oxide anion (O2-).
Valency
The combining power (or capacity) of an element is called its valency. Valency can be used to find out how the atoms of an element will combine with the atom(s) of another element to form a chemical compound.
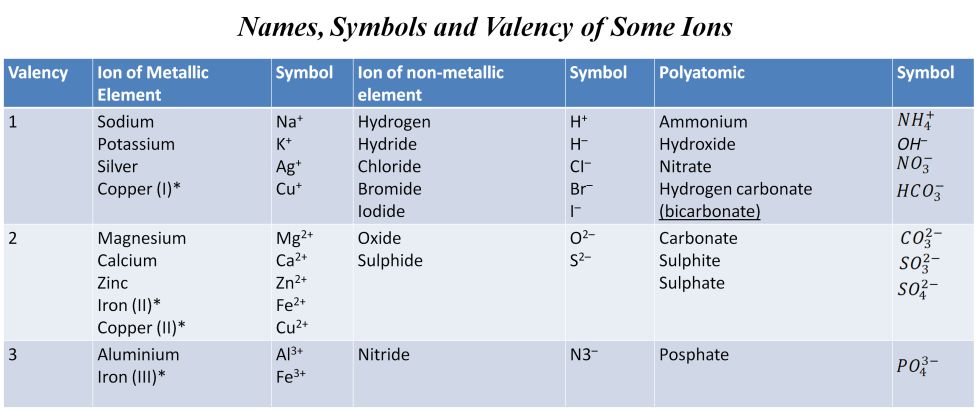
Writing Chemical Formulae 1
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Writing Chemical Formulae
The shortest way to represent a compound with the help of symbols and valency of elements is known as chemical formula. Chemical formula of a compounds shows of each combining element.
In ionic compounds, the charge on each ion is used to determine the chemical formula of a compound.
There are some rules for writing the chemical formula
(i):- The valencies or charges on the ion must be balanced.
(ii):- When a compound consists of a metal and a non-metal, the symbol of the metal is written first and on the left whereas of non-metal on its right.
(iii):- When compound is formed with polyatomic ions, the ion is enclosed in a bracket before writing the number to indicate the ratio. e.g. Ca(OH)2.
Formulae of Simple Compounds
To write the chemical formula for simple compounds.
(i):- Write the symbols of constituent elements and their valencies as shown below.
(ii):- Write the symbol of cation first followed by the symbol of anion.
(iii):- Then criss-cross their charges or valencies to get the formula.
Note:- The simplest compounds made up of two different elements are also called binary compounds.
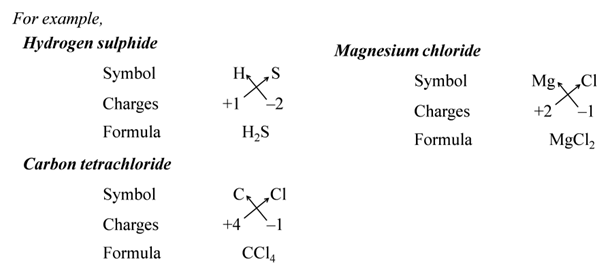
In other words, the positive and negative charges must balance each other and the overall structure must be neutral.
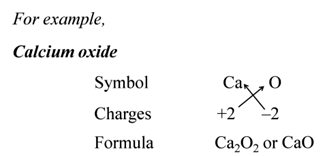
Note:- When the valency of both elements are numerically equal, the subscripts are also not written.
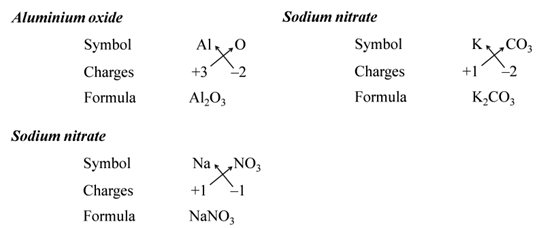
We use brackets when we have two or more of the same ions in the formulae. For Example:-
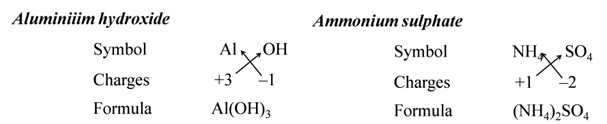
All subscripts must be reduced to lowest term (except for molecule or covalent compound). For Example:-

Illustration
Write symbols of the following elements:
Silver, Chromium, Chlorine, Mercury, Lead, Copper, Gold, Aluminium.
Solution
Silver (Ag), Chromium (Cr), Chlorine (CI), Mercury (Hg), Lead (Pb), Copper (Cu), Gold (Au), Aluminium (Al).
Illustration
Define atomic mass unit.
Solution
One atomic mass unit is the mass unit (u) equal to exactly 1/12th of the mass of one atom of C-12 isotope.
Illustration
How many kinds of atoms are present in a molecule of calcium carbonate (CaCO3)?
Solution
CaCO3 is a heteroatomic molecule which contains three types of atoms, i.e. one atom of calcium, one atom of carbon and three atoms of oxygen.
Molecular Mass
The molecular mass of a substance is the sum of the atomic Masses of all the atoms in a molecule of the substance. Therefore, the relative molecular mass of a molecule is its relative mass expresses in atomic mass units (u).
For example,
The relative molecular mass of water (H2O) is 18 u, which can be calculated as
Atomic mass of hydrogen =1 u
Atomic mass of oxygen = 16 u
H2O contains two hydrogen atoms and one oxygen atom. Therefore, molecular mass of water is
= 2x1 + 1 x 16 = 18 u
Illustration
Calculate the molar mass of the following substances:
(i):- Ammonia, (ii):- Hydrochloric acid, (iii):- Phosphorus molecule,
(iv):-Hydrogen molecule, (v):- Oxygen molecule, (vi):- Sulphur dioxide
Solution
(i):- Molar mass of ammonia (NH3)
=1 x 14 + 3 x 1 = 17 u
(ii):- Molar mass of hydrochloric acid (HCI)
=1 x 1 + 1 x 35.5 = 36.5 u
(iii):- Molar mass of phosphorus molecule (P4)
= 4 x 31 = 124 u
(iv):- Molar mass of hydrogen molecule (H2)
= 2 x 1= 2 u
(v):- Molar mass of oxygen molecule (O2)
= 2 x 16 = 32 u
(vi):- Molar mass of sulphur dioxide (SO2)
= 32 + 2 x 16 = 64 u
Formula Unit Mass
It is the sum of the atomic masses of all atoms present in a formula unit of a compound.
Formula unit mass is calculated in the same manner as we calculate the molecular mass.
e.g. formula unit mass for sodium chloride (NaCI)
= 1 x 23 + 1 x 35.5 = 58.5 u
Mole Concept
One mole of any species (atoms, molecules, ions or particles) is that quantity in number having a mass equal to its atomic or molecular mass in grams.
e.g. 1 mole of carbon (atomic mass =12) is equal to 12 g.
1 mole of oxygen (O2, molecular mass = 2 x 16) is equal to 32 g.
1 mole of water (H2O, molecular mass = 2 x 1 + 1 x 16) is equal to 18 g.
Avogadro Constant
The number of particles present in 1 mole of any substance is same and fixed, which is equal to 6.022 x 1023. This is a constant, known as Avogadro constant or Avogadro number (NA).
Thus, mole is also defined as number of particles equal to the Avogadro constant, NA(6.022 x 1023).
1 mole = 6.022 x 1023 particles, in number.
Molar Mass and Moles
The mass of 1 mole of a substance is equal to its relative atomic or molecular mass in gram. Since the atomic mass or molecular mass of an element gives us the mass of one atom of that element in atomic mass units (u). Thus, to get the mass of 1 mole of an atom of that element we have to take the same numerical value but change the units from `if to 'g‘.
Illustration
Find the mass of :
(i):- 1 mole of nitrogen atoms, (ii):- 8 moles of aluminium atoms, (iii):- 0.2 mole of oxygen atoms, (iv):-2 moles of water molecules.
Solution
(i):- Mass of 1 mole of nitrogen atoms
- = molar mass in gram =14 g
(ii):- We know that,
- mass of 1 mole of Al-atoms = 27 g
- Mass of 8 moles of Al-atoms = 8 x 27 = 216 g
(iii):- Mass of 0.2 mole of oxygen atoms = 0.2 x 16 = 3.2 g
- Mass of 1 mole of oxygen atoms =16 g
(iv):- Mass of 2 moles of water molecules = 2 x 18 = 36 g
- Mass of 1 mole of water molecule =18 g
Percentage Composition
The percentage composition of an element in a compound is the percentage of the mass contributed by the element to the total mass of the compound. It is obtained by dividing mass of that element in the compound by the total mass of the compound and multiplying by 100, i.e.,.
![]()
Illustration
Calculate the percentage composition of carbon in CO2.
Solution
Molar mass of CO2 =12 + 2 x16 = 44g mol-1
Mass due to carbon (C) = 12 g
Percentage composition of C = 12/44 x100 = 27.3%
Advanced Level
Illustration
Write the molecular formulae of all the compounds that can be formed by the 3M combination of the following ions: ![]()
Solution
(a):- Compounds of Cu2+ = CuCl2, CuSO4, Cu3(PO4)2
(b):- Compounds of Na+ = NaCl, Na2SO4, Na3PO4
(c):- Compounds of Fe3+ = FeCl3, Fe2(SO4)3, FePO4
Illustration
Calculate the number of moles of magnesium present in a magnesium ribbon weighing 12 g. Molar atomic mass of magnesium is 24 g mol-1.
From the table, find
(a) A pair of ions (b) An atom of noble gas
(c) A pair of isobars (d) A pair of isotopes
Solution
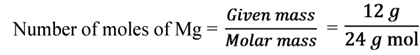
Illustration
Calculate the formula unit mass of CaCl2.
Solution
Atomic mass of Ca = 40 and Cl = 35.5
CaCl2 = 40 + 2 x 35.5 = 40 + 71 = 111u
Illustration
Give the names of any two elements present in the following compounds:
Baking powder, Common salt, Sulphuric acid
Solution
Baking powder (NaHCO3) Sodium, hydrogen, carbon and oxygen.
Common salt (NaCl) Sodium and chlorine.
Sulphuric acid (H2SO4) Sulphur, hydrogen and oxygen.
Illustration
6 g of coke consisting 100% carbon, is burnt in air. Find the number of oxygen atoms consisted by the carbon dioxide gas thus formed.
Solution
Each carbon atom combine with two oxygen atoms up on combustion as follows: 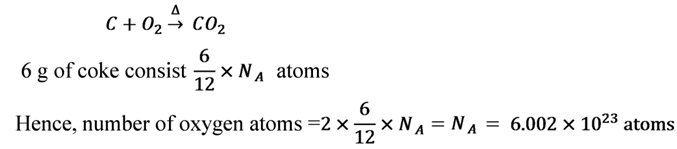
Illustration
Find the number of atoms in 120 g of calcium and 120 g of iron. Which one has more number of atoms and how much is the difference?
[Atomic mass of Ca = 40 u, Fe = 56 u]
Solution
Molar mass of Ca = 40 g
∵ 40 g of Ca contains, number of atoms = 6.022 x 1023
Then, let the size of the nucleus be x m.
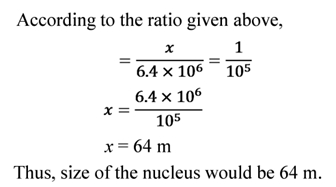
Illustration
List down three different names given to the path in which electrons revolve around the nucleus. Also explain why are they called so?
Solution
The three different names are:-
Discrete orbit It is called so because electrons revolve in certain distinct path and not just in orbit.
Energy level (energy shell) The energy associated with different orbits (with which electron revolves) is distinct for each orbit, hence it is called so.
Stationary state Since, the energy associated with an orbit is fixed. Hence, the electron revolving in a particular orbit have stationary energy. That's why, orbit is also called stationary state.
Illustration
A metal (mass number = 40) having same number of protons and neutrons, combines with two chlorine atoms. Identify the element with which electronic configuration of this metal matches in combined state.
Solution
∵ Mass number = Number of protons + Number of neutrons
⇒ 40 = 2 x Number of protons
⇒ Number of protons = 20
Hence, the metal is calcium (Ca).
Since, it combines with 2 Cl atoms (total valency = 2)
Hence, it will acquire 2 positive charge, after losing 2 electrons as
K L M
Electronic configuration of Ca2+ ion = 2, 8, 8
This electronic configuration matches to that of argon (Ar).
Discovery of Electron
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 4
Structure of The Atom
Charged Particles in Matter
Some charged particles are present within the atom or atom is made up of some charged Two such particles are electrons and protons.
Discovery of Electrons
The electron, was identified by JJ Thomson, when he performed cathode ray experiment using a discharge tube. In this experiment, he found a beam of negatively charged particles, called the cathode rays, as they were originated from the cathode.
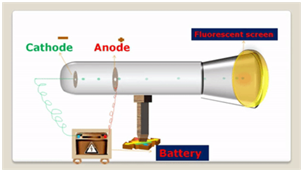
The Characteristics Exhibited by Cathode Rays are
- These rays travel in a straight line and rotate a pin wheel placed in their path. This shows that these consist of material particles possessing high kinetic energy.
- In the presence of electric field, these get deflected towards the positive electrode. This shows that these rays are made up of negatively charged particles.
- Millikan found the charge on electron (1.6 x 10–19C).
- Mass of electron is (9.1 x 10–31kg).
Discovery of Proton
E Goldstein in 1886, discovered the presence of new radiations known as canal rays or anode rays. These are the positively charged rays which are seen moving from the anode towards cathode in a specially designed discharge tube when a high voltage is applied across the electrodes. Porous cathode is used to provide the path for passing anode rays. It led to the discovery of another sub-atomic particle, the proton (1.602 x 10–19C).
Some Characteristics Features of Anode Rays are
- These rays always travel in a straight line.
- Anode rays get deflected by electric and magnetic fields in a direction opposite to the cathode rays.
- These consist of positively charged particles, known as protons.
- Proton had a charge, equal in magnitude but opposite in sign to that of electron. Its mass was 1840 times. i.e., approximately 2000 times, as that of the electron.
- The e/m values for the particles are not the same for all the gases enclosed in the discharge tube.
- The nature of anode rays depends upon the nature of gas enclosed in the discharge tube.
Conclusion
In general, an electron is represented as e– and a proton as p+. The mass of a proton is taken as one unit and its charge as plus one (+1), where as the mass of an electron is considered to be negligible and its charge is minus one (–1). It seemed likely that an atom was composed of protons and electrons, mutually balancing their charge.
The Structure of an Atom
According to Dalton’s atomic theory, atom was indivisible and inderstuctible. The discovery of two fundamental particles inside the atom, led to the failure of this aspect of Dalton’s Theory. To know the arrangement of electrons and protons within an atom, many scientists proposed various atomic models.
Thomson’s Model of an Atom
Thomson's model of an atom was similar to watermelon. The electrons in a sphere of positive charge, were like seeds in a spherical watermelon. In which, the positive charge in an atom is spread all over like the red edible part, while the electrons studded in the positively charged sphere, like the seeds in the watermelon.

Following are The Postulates of This Model
- Electrons are embedded in the sphere of positive charge.
- The negative and positive charges balance each other. Therefore, atom as a whole is neutral.
- Mass of an atom is due to the electrons.
Limitations of Thomson's Model of an Atom
- JJ Thomson's model could not explain the experimental results of other scientists, as there is no nucleus in the atomic model proposed by Thomson.
- It does not have any experimental evidence in its support.
Rutherford's Model of an Atom
Ernest Rutherford bombarded thin sheets of gold foil with fast moving α-particles (These are doubly charged helium ions having a mass of 4 u). He selected a gold foil because he wanted a layer as thin as possible. Thin gold foil was about 1000 atoms thick.
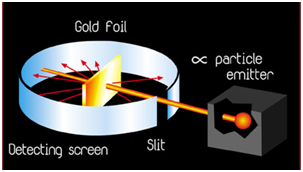
The Following Observations Were Made By Rutherford
- Most of the fast moving α -particles passed straight through the gold foil.
- Some of the α -particles were deflected by the foil by small angles.
- Very few α -particles (one out of 12000) appeared to rebound.
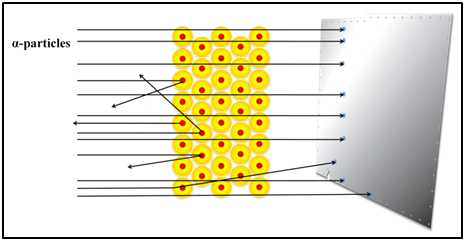
On the basis of his experiment, Rutherford put forward the nuclear model of an atom, having the following features
- There is a positively charged, highly densed centre in an atom, called the nucleus. Nearly, the whole mass of the atom resides in the nucleus.
- The electrons revolve around the nucleus.
- The size of the nucleus (10 -I5 m) is very small as compared to the size of the atom (10-10 m).
Limitations of Rutherford's Model of an Atom
- Any charged particle when accelerated is expected to radiate energy. Thus, the revolving electron would lose energy and finally fall into the nucleus. If this were so, the atom should be highly unstable.
- Rutherford's model could not explain the distribution of electrons in the extra nuclear portion of the atom.
Bohr's Model of an Atom
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Bohr's Model of an Atom
To overcome the objections raised against Rutherford's model of the atom, Neils Bohr put forward the following postulates about the model of an atom.
(i):- Atom consists of positively charged nucleus around which electrons revolve in discrete orbits i.e., electrons revolve in certain permissible orbits and not just in any orbit.
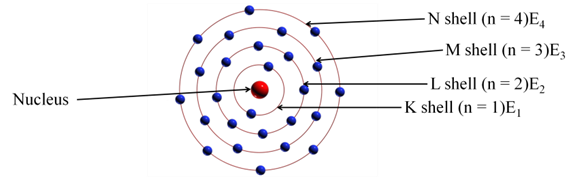
(ii):- Each of these orbits are associated with certain value of energy. Hence, these orbits are called energy shells or energy levels.
(iii):- Starting from nucleus, energy levels (orbits) are represented by numbers (1, 2, 3, 4, etc) or by alphabets (K, L, M, N, etc).
(iv):- Normally, the electrons present in first energy level (E1) have lowest energy. k Energies increase going towards outer energy levels.
(v):- Energy of an electron remains same as long as it remains in discrete orbit and it does not radiate energy while revolving.
(vi):- When energy is supplied to an electron, it can go to higher energy levels. While an electron falls to lower energy level, it radiate energy.
Neutrons (n)
Neutrons are another sub-atomic particles, discovered by J Chadwick in 1932. It is represented by n. Neutrons are electrically neutral particles and are as heavy as protons.
(i.e., their mass is 1.67493x10–27 kg). Neutrons are present in the nucleus of all atoms except hydrogen. The mass of the atom is given by the sum of the masses of protons and neutrons present in the nucleus.

Distribution of Electrons in Different Orbits (Shells)
For writing the number of electrons in different energy levels, there are some rules.
- The maximum number of electrons present in a shell is given by the formula 2n 2, where n is the orbit number or energy level, 1, 2, 3…….
Therefore, the maximum number of electrons in different shells are as follows
First orbit or K-shell = 2 x (1)2 = 2
Second orbit or L-shell = 2 x (2)2 = 8
Third orbit or M-shell = 2 x (3)2 =18
Fourth orbit or N-shell = 2 x (4)2 = 32 and so on.
- The maximum number of electrons that can be accommodated in the outermost orbit is 8.
- Electrons are not accommodated in a given shell, unless the inner shells are filled.
Valency
The electrons present in the outermost shell of an atom are known as the valence electrons. The combining capacity of the atom of an element with the atom(s) of other element(s) in order to complete its octet is known as valency.
Atomic Number
It is defined as the number of protons present in the nucleus of an atom. It is nearly denoted by Z.
For Example:-
![]()
Mass Number
It is defined as the sum of number of protons and neutrons present in the nucleus of an atom. Mass number is denoted by A.
Mass number (A) = Number of protons + Number of neutrons
![]()
Number of neutrons = Mass number – Atomic number
∵ Atomic number = Number of protons
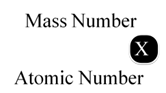 In the notation for an atom, the atomic number, mass number and symbol of the element are to be written as
In the notation for an atom, the atomic number, mass number and symbol of the element are to be written as
Atomic Weight
Mass of an individual atom is called atomic mass or atomic weight. It is nearly equal to the mass number.
For Example:-
(i):- Carbon, atomic number (Z) = 6,
Mass number (A) =12,
Atomic mass =12.01 u
(ii):- Oxygen, atomic number (Z) = 8,
mass number (A) = 16, atomic mass = 15.99
Different Atomic Species; Isotopes
These are defined as the atoms of the same element, having the same atomic number but different mass numbers. For Example:- there are 3 isotopes of hydrogen atom, namely protium ![]()
![]() and tritium
and tritium ![]()
For Example:- The two isotopic forms of chlorine atom with masses 35 u and 37 u occur in the ratio of 3 : 1.
Therefore, the average atomic mass of chlorine atom, can be calculated as
![]()
Applications of Isotopes
- An isotope of uranium (U-235) is used as a fuel for the production of electricity in nuclear reactors.
- U-238 is used to determine the age of very old rocks and even the age of the earth.
- An isotope of cobalt (Co-60) is used in the treatment of cancer.
- An isotope of carbon (C-14) is used to determine the age of old specimens of wood or old bones of living organisms.
- An isotope of iodine (I-131) is used in the treatment of goitre.
- P-32 is used in agricultural research.
- Na-24 is used to detect blood clots.
Isobars
Atoms of different elements with different atomic numbers, but same mass number, are known as isobars.
![]()
Isotones
Atoms of different elements having the same number of neutrons are called isotones.
![]()
Change of state of matter
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Change of state of matter
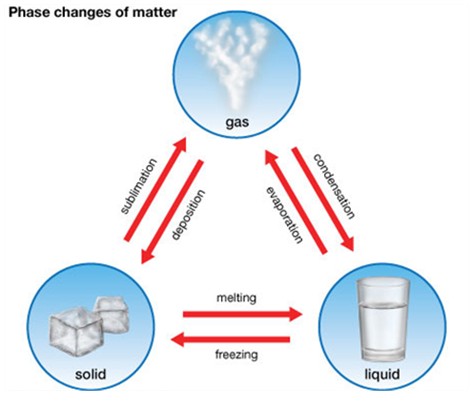
We can change the physical state of matter in two ways:
- By changing the temperature, and
- By changing the pressure.
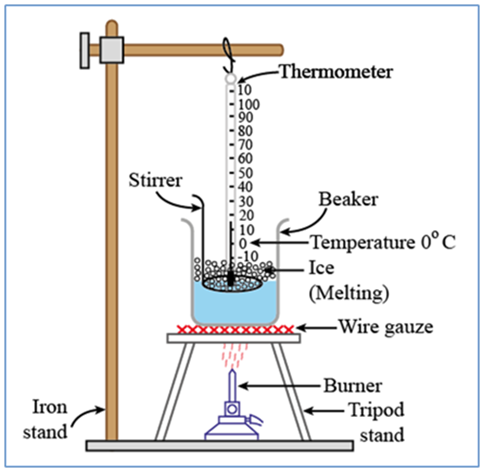
Solid to Liquid Change: Melting
The process, in which a solid substance changes into a liquid on heating, is called melting (or fusion).
Liquid to Gas Change: Boiling (or Vaporization)
Place during boiling. The boiling of a liquid takes place at a fixed temperature. The temperature, at which a liquid boils and changes rapidly into a gas at atmospheric pressure, is called boiling point of the liquid.
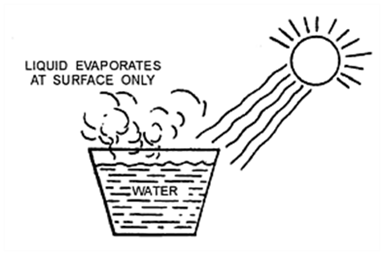
When a liquid is heating, it changes its physical state and becomes a gas.
Gas to Liquids Change:- Condensation
The process of changing a gas to liquid by cooling is called condensation.

Liquid to Solid Change: Freezing
The process of changing a liquid into a solid by cooling is called freezing.
When a liquid is cooled its particles lose energy due to which they move slowly. At this stage the liquid freezes and becomes a solid.
Suspension and Evaporation
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Suspension and Evaporation
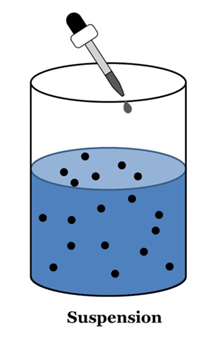
Suspension
A suspension is a heterogeneous mixture in which the solute particles do not dissolve but remain suspended throughout the bulk of the medium.
Properties of a Suspension
- Suspension is a heterogeneous mixture.
- Its particles can be seen by naked eyes.
- Its particles scatter a beam of light passing and make its path visible (Tyndall effect).
- It is unstable. The soluble particles settle suspension is left undisturbed or they can by the process of filtration.
Colloidal Solution

A colloid (or colloidal solution) is a mixture that is heterogeneous but appears to be homogeneous as the articles are uniformly spread
throughout the solution. e.g., milk, shaving cream, cheese etc.
Properties of a Colloid
- A colloid appears to be homogeneous but actually it is heterogeneous.
- A colloid appears to be homogeneous but actually it is heterogeneous.
- The size of particles of colloid is very small. They can not be seen even with a microscope.
- Its particles can pass through filter paper, therefore, a colloid cannot be separated by filtration. However, they get separated by a special technique, called centrifugation.
Some Common Examples of Colloids
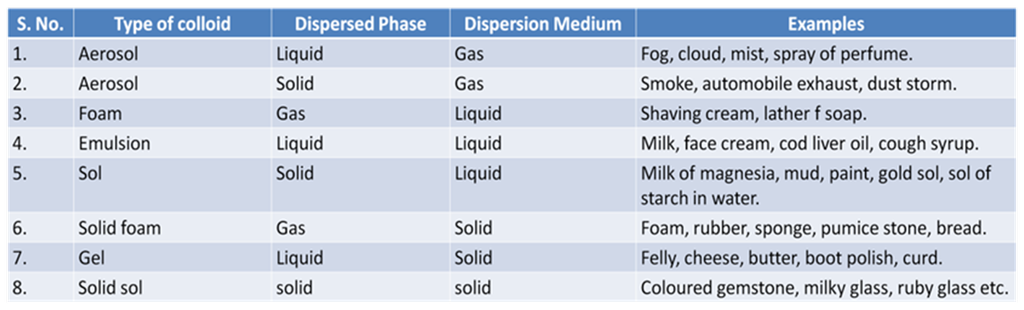
Components of a Colloid
The solute like component form the dispersed phase and the component in which the dispersed phase is suspended is known as the dispersion medium.
Evaporation
This method can be used to separate a volatile component (solvent) from a non-volatile component of a mixture.
Centrifugation
(Separation of Cream from Milk)
Two components having difference in densities can be separated by centrifugation.
Decantation
This method is also applied where a difference between densities of components lies. A less soluble solid from a liquid can be separated by first keeping the mixture undisturbed and then decanting the liquid slowly leaving the solid in the first container.
Separating Funnel
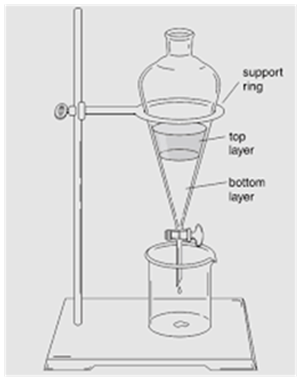
(Separation of Mixture of Two Immiscible Liquids)
This method is used for separating two immiscible liquids and is also based on the difference in densities.
Dalton's Atomic Theory
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Dalton's Atomic Theory
Dalton's atomic theory provided an explanation for the law of conservation of mass and the law of definite proportions. According to Dalton’s atomic theory, all matter (whether an element, a compound or a mixture), is compounds of small particles, called atoms.
The Main Postulates of Dalton's Atomic Theory are
- Every matter is made up of very small particles, called the atoms.
- Atoms are indivisible particles which can neither be created nor be destroyed in a chemical reaction.
- Atoms of a given element are identical in mass as well as in chemical properties.
- Atoms of different elements have different masses and chemical properties.
- Atoms combine in the ratio of small whole numbers to form compounds.
- The relative numbers as well as kinds of atoms are constant in a given compound.
Merits of Dalton's Atomic Theory
Dalton's atomic theory has proved to be very useful in various ways
- This theory has enabled us to explain the laws of chemical combination.
- Dalton was the first to recognize a workable distinction between the ultimate particle of an element (atom) and that of a compound (molecule).
Demerits of Dalton's Atomic Theory
- Atom is no longer considered as the smallest indivisible particle. It has been established that it is made up of electrons, protons and neutrons.
- Atoms of the same element may have slightly different masses (Isotopes).
- Atoms of different elements may have the same masses (Isobars).
- Substance made up of the same kind of atoms may have different properties.
- The ratio in which the different atoms combine to form compound may be fixed and integral but may not be simple.
Atoms
Atoms are the smallest particles of an element which may or may not have independent existence but take part in a chemical reaction. These are the building blocks of all natter.
For Example:- atoms of hydrogen, oxygen, nitrogen, etc, are not capable in independent existence whereas atoms of helium, noon, etc are capable of independent existence.
Size of Atoms
Atoms are very small and their radius is measured in nanometres.
1/109 M =1 nm or lm = 109 nm
Radius of hydrogen atom is 0.1 nm.
Modern Symbols of Atoms of Different Elements
Although Dalton was the scientist who introduced symbols for representing elements for the first time but modern symbols for the elements were introduced by JJ Berzilius. These are defined as a short hand representation of the name of an element.
Now a days, it is the IUPAC who approves the names of the letters of the element’s name in English. The first letter of a symbol is always written in capital letter and the second letter as small letter.
e.g., chlorine – Cl, zinc – Zn and aluminium – Al.
Symbols of some elements have been taken from their names in different languages such as Latin, German, Greek, etc.
For Example:-
- Iron – Fe from Ferrum (Latin Name)
- Gold – Au from Aurum (Latin Name)
- Potassium – K from Kalium (Latin Name)
- Chlorine – Cl from Chloros (Greek Name)
- Cobalt – Co from Kobold (German Name)
- Sodium– Na from Natrium (Latin Name)
Atomic Mass
According to Dalton, each element has a characteristic atomic mass. But determining the mass of an individual atom was a relatively difficult task due to its very small size.
Hence, their relative atomic masses were determined using the laws of chemical combination and the compounds formed. For this purpose, initially oxygen was taken as standard because of the following two reasons:
(i):- Oxygen reacted with a large number of elements and formed compounds.
(ii):- This unit gave masses of most of the elements as whole numbers.
Relative Atomic Mass
It is defined as the number of times a given atom is heavier than 1/12th of mass of 1 atom of carbon-12 (C-12) or it is the average mass of the atom as compared to 1/12th the mass of one carbon-12 atom.
Atomic Mass Unit
It is defined as the mass unit equal to exactly 1/12th of the mass of one atom of C-12 isotope. Earlier, it was abbreviated as amu but according to latest recommendations of IUPAC, it is now written as `u'— unified mass
Writing Chemical Formulae
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Writing Chemical Formulae
The shortest way to represent a compound with the help of symbols and valency of elements is known as chemical formula. Chemical formula of a compounds shows of each combining element.
In ionic compounds, the charge on each ion is used to determine the chemical formula of a compound.
There are some rules for writing the chemical formula
(i):- The valencies or charges on the ion must be balanced.
(ii):- When a compound consists of a metal and a non-metal, the symbol of the metal is written first and on the left whereas of non-metal on its right.
(iii):- When compound is formed with polyatomic ions, the ion is enclosed in a bracket before writing the number to indicate the ratio. e.g. Ca(OH)2.
Formulae of Simple Compounds
To write the chemical formula for simple compounds.
(i):- Write the symbols of constituent elements and their valencies as shown below.
(ii):- Write the symbol of cation first followed by the symbol of anion.
(iii):- Then criss-cross their charges or valencies to get the formula.
Note:- The simplest compounds made up of two different elements are also called binary compounds.
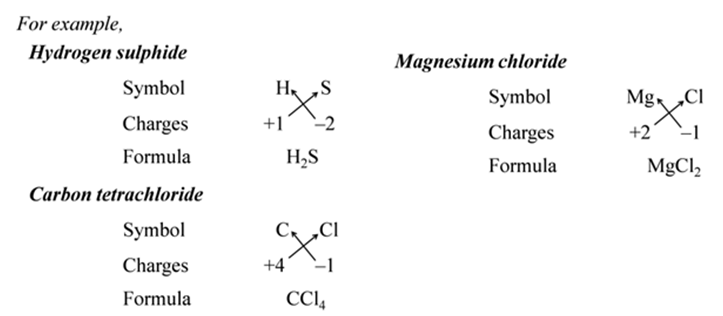
In other words, the positive and negative charges must balance each other and the overall structure must be neutral.
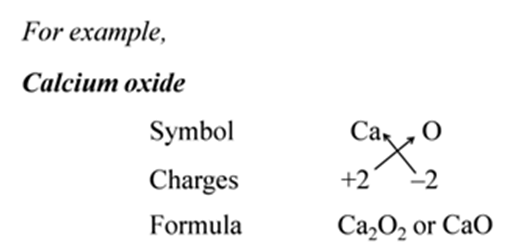
Note:- When the valency of both elements are numerically equal, the subscripts are also not written.
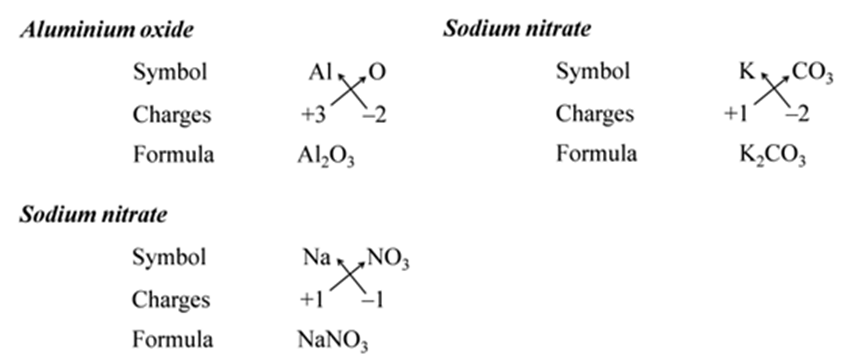
We use brackets when we have two or more of the same ions in the formulae. For Example:-
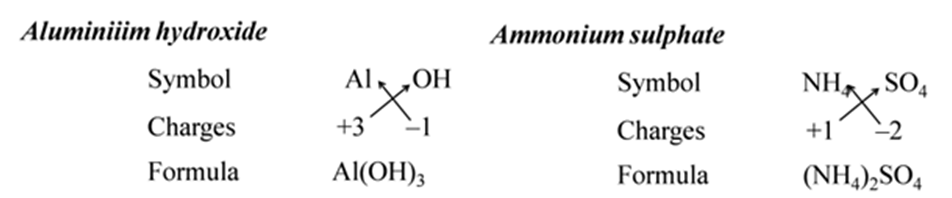
All subscripts must be reduced to lowest term (except for molecule or covalent compound). For Example:-

Molecular Mass
The molecular mass of a substance is the sum of the atomic Masses of all the atoms in a molecule of the substance. Therefore, the relative molecular mass of a molecule is its relative mass expresses in atomic mass units (u).
For example,
The relative molecular mass of water (H2O) is 18 u, which can be calculated as
Atomic mass of hydrogen =1 u
Atomic mass of oxygen = 16 u
H2O contains two hydrogen atoms and one oxygen atom. Therefore, molecular mass of water is
= 2x1 + 1 x 16 = 18 u
Formula Unit Mass
It is the sum of the atomic masses of all atoms present in a formula unit of a compound.
Formula unit mass is calculated in the same manner as we calculate the molecular mass.
e.g. formula unit mass for sodium chloride (NaCI)
= 1 x 23 + 1 x 35.5 = 58.5 u
Mole Concept
One mole of any species (atoms, molecules, ions or particles) is that quantity in number having a mass equal to its atomic or molecular mass in grams.
e.g. 1 mole of carbon (atomic mass =12) is equal to 12 g.
1 mole of oxygen (O2, molecular mass = 2 x 16) is equal to 32 g.
1 mole of water (H2O, molecular mass = 2 x 1 + 1 x 16) is equal to 18 g.
Avogadro Constant
The number of particles present in 1 mole of any substance is same and fixed, which is equal to 6.022 x 1023. This is a constant, known as Avogadro constant or Avogadro number (NA).
Thus, mole is also defined as number of particles equal to the Avogadro constant, NA(6.022 x 1023).
1 mole = 6.022 x 1023 particles, in number.
Molar Mass and Moles
The mass of 1 mole of a substance is equal to its relative atomic or molecular mass in gram. Since the atomic mass or molecular mass of an element gives us the mass of one atom of that element in atomic mass units (u). Thus, to get the mass of 1 mole of an atom of that element we have to take the same numerical value but change the units from `if to 'g‘.
Percentage Composition
The percentage composition of an element in a compound is the percentage of the mass contributed by the element to the total mass of the compound. It is obtained by dividing mass of that element in the compound by the total mass of the compound and multiplying by 100, i.e.,.
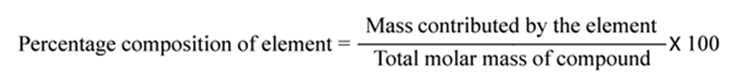

 Kaysons Publication
Kaysons Publication
