- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Ethanoic Acid or Acetic Acid
It is commonly known as acetic acid. Its formula is CH3COOH. 5-8% solution of ethanoic acid in water is known as vinegar.
Physical Properties
- Its melting point is 290K.
- During winters it often freezes and form ice like flakes, so it is also called glacial acetic acid.
Chemical Reactions
Acidity It is a weaker acid than HCl but stronger than alcohol. It evolves hydrogen when reacts with sodium metal or sodium hydroxide, which shows its acidic nature.
2CH3COOH + 2Na → 2CH3COONa + H2 ↑
CH3COOH + NaOH → CH3COONa + H2O ↑
Esterification When ethanol (an alcohol) reacts with acetic acid (a carboxylic acid), a fruity (sweet) smelling liquid called ester is obtained. This reaction is called esterification.
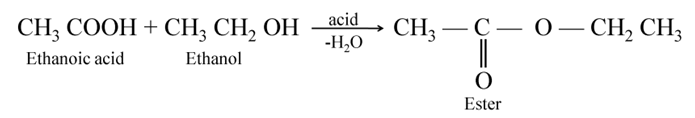
Saponification
The ester gets converted back into alcohol and sodium salt of acid when treated with alkali like sodium hydroxide. This reaction is called saponification, as it is used for the preparation of soap.
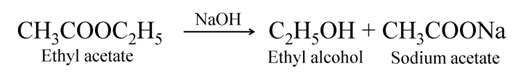
Reaction with carbons and hydrogen carbonates in the reaction of acetic acid with carbonates or hydrogen carbonates, carbon dioxide gas is obtained. It is an example of acid-base reaction.
![]()
![]()
Uses of Acetic Acid
- It is used for making vinegar.
- It is widely used as a preservative in pickles.
- It is used for the synthesis of other compounds like esters.
Soaps and Detergents
Soaps are sodium or potassium salts of long chain carboxylic acids and have general formula RCOONa.
Detergents are usually ammonium or sulphonate salts of long chain carboxylic acids.
Manufacture of Soaps and Detergents
Soaps are made from animal fat or vegetable oils by heating it with sodium hydroxide.
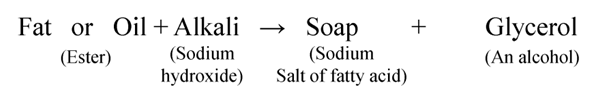
This process of preparation of soap by the hydrolysis of fats and oil with alkalies is called saponification.
Structure of a Soap Molecule
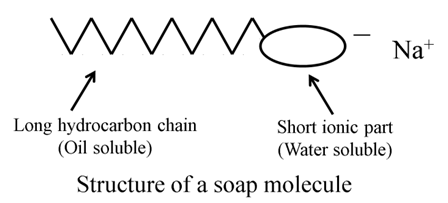
A soap molecule is made up of two parts — a long hydrocarbon part and a short ionic part containing — COONa group. The long hydrocarbon part is hydrophobic and therefore insoluble in water but soluble in oil. The ionic portion of the soap molecule is hydrophilic so, soluble in water and insoluble in oil.
Cleansing Action of Soaps (Micelle Formation)
Some molecules have different properties at their two ends. Its one end is hydrophilic (soluble in water) and other is hydrophobic (soluble in fats or oils).
Inside water these molecules show a unique orientation that keeps the hydrocarbon portion out of the water. This is done by forming clusters of molecules in which the hydrophobic tails are in the interior of the cluster and on the surface of cluster ionic ends are present. This formation is called micelle formation.
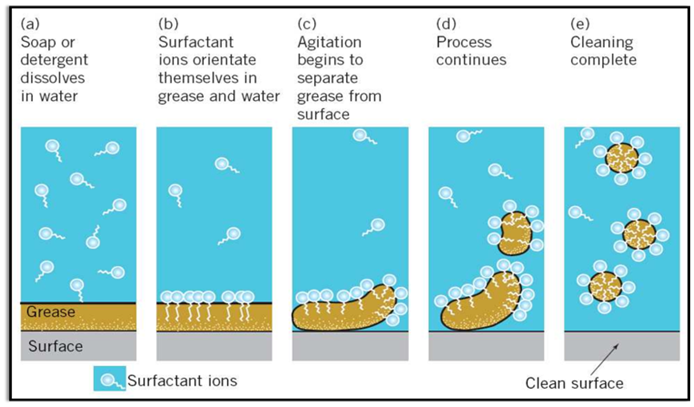
Limitations of Soap
Soap is not suitable for washing clothes with hard water. Because hard water contains calcium and magnesium salts. Soap forms scum with hard water as it reacts with calcium and magnesium ions present in the hard water.
Detergents are used as cleansing agents in case of hard water become charged ends of detergents do not form insoluble precipitates with magnesium and calcium ions present in hard water. Hence, they are effective in hard water.
Detergents are useful in making shampoos and products for cleaning clothes but their main disadvantage is that these are generally non-biodegradable.
Advantages of Detergents Over soaps
Synthetic detergents do not require vegetable oil or fats for their preparation, hence they help in saving oils and fats for human consumption.
Synthetic detergents can be used with hard water while soaps cannot be used with hard water.

 Kaysons Publication
Kaysons Publication
