- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Modern Periodic Table and Various Trends in it
In 1913. Henry Moseley showed that the atomic number of an element is' a more fundamental property and on the basis of this, he modified the Mendeleev's periodic law as “Physical and chemical properties of the elements are a periodic function of their atomic number”. This is called modern periodic law.
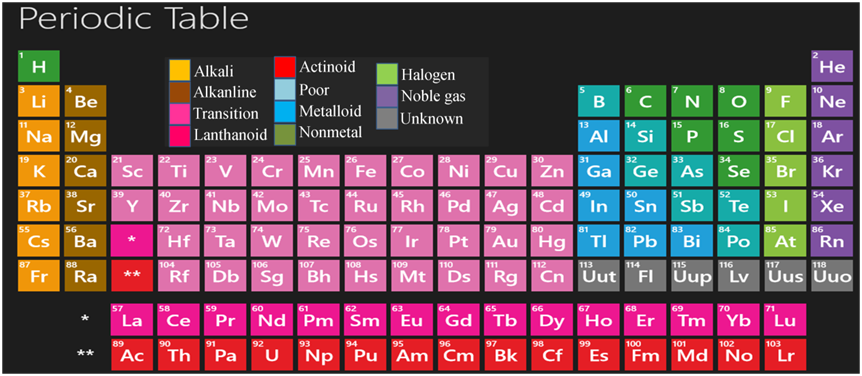
Features of Modern Periodic Table
This table has 18 vertical columns, known as groups and 7 horizontal rows, known as periods.
Features of Groups
- A few important features of the elements present in groups are as follows.
The elements present in a group are separated by definite gaps of atomic numbers.
- The groups are not divided into sub-groups.
- The elements present in a group have the same number of electrons in the valence shell of their atoms or same outermost electronic configuration.
- The elements present in a group have the same valiancy.
- The elements present in a group have identical chemical properties.
- The number of shells increases as we go down the group.
- The elements present in the group 18 are almost chemically uncreative. These are also called noble gas elements.
Features of Periods
The important features of the elements in a period are as follows:-
- Elements of a period do not have the same number of valence electrons but they contain the same number of shells.
- The number of valence shell electrons increase by one unit as the atomic number increases by one unit on moving from left to right in a period.
- As the number of valence shell electrons changes, the chemical properties of the elements also change
- Different periods have different number of electrons which can be explained on the basis of filling of electrons into various shells.
Position of the Elements in the Modern Periodic Table
- In order to find the position of an element in the periodic table, first write its electronic Configuration and then find period and group number as follows.
- The period number of an element is equal to the number of electron shells in its atom.
For Example, if the atom of an element has 2 electron shells (K and L), then it belongs to 2nd period.
- If two (or more) elements have the same number of valence shells, then they belong to the same period of the periodic table.
- The group number of an element having up to two valence electrons is equal to the number of valence electron.
Explanation of the Anomalies of Mendeleev's Periodic Table
Different anomalies of Mendeleev’s table can be explained with the help of modern periodic table as
- The fundamental basis for modern periodic table is atomic number, not atomic mass, hence it is more accurate.
- Since, the table is based on atomic number and isotopes have same atomic number and chemical properties, so they can be put at one place in the same group of the periodic table.
- In this periodic table, a unique, position has been given to the hydrogen. It is kept at the top left corner because of its unique characteristics.
- The position of cobalt and nickel is justified itself because atomic number of cobalt is less than atomic number of nickel.
- A separate group for noble gases was created when these were discovered.
Trends in the Modern Periodic Table
In this table, some properties show a regular trend when we move along a period from left to right or in a group from top to bottom.
These properties are explained in next topics
Valency
It is the combining capacity of an atom of an element to acquire noble gas configuration and depends upon the number of valence electrons the electrons present in the outermost shell.
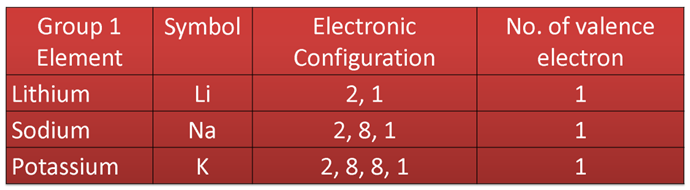
Variation Along a Group
In a group, outer electronic configuration is same for all the elements, so all have the same number of valence electrons or the valency.
e.g. in case of elements of group 1
Similarly, for the elements of group 17

Variation Along a Period
Since, the number of valence electrons increases one by one from left to right along a period, so valency also increases from 1 to 7 with respect to oxygen. While with respect to hydrogen it first increases from 1 to 4 and then decreases to 0.

Atomic Size
It refers to the radius of an atom and is defined as the distance between the centre of the nucleus and the outermost shell of an isolated atom.
Variation Along a Period
The atomic radius decreases on moving from left to right along a period. This is due to an increase in nuclear charge which tends to pull the valence electrons closer to the nucleus and reduces the size of the atom
Variation Along a Group
The atomic size increases down the group. This is because new shells are being added as we go down the group. This increases the distance between the outermost electrons and the nucleus.

 Kaysons Publication
Kaysons Publication
