- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 2
Acids, Bases and Salts
Introduction
All the compounds on the basis of their chemical properties can be classified as acids, bases and salts. They have certain definite properties which distinguish one compound class from the other. The sour taste of many fruits and vegetables, (e.g., lemon, orange, amla, etc) is due to various types of acids present in them. The digestive fluids of most humans and animals contain acids. The bitter taste of substance like bitter gourd, cucumber extract, etc is due to bases present in them.
Acids
The word acid comes from the Latin word acidus acere which mean sour. Acids are those chemical substances which have a sour taste and turn blue litmus solution red. Some common fruits such as raw mango, lemon, orange, tamarind, etc are sour in taste.
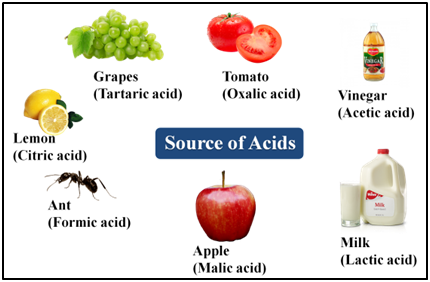
Classification of Acids
- On the Basis of Source
- On the Basis of Ionisaion
- On the Basis of the Amount/Concentration of Acid present in Aqueous Solution
On the Basis of Source
(i):- Inorganic Acids or Mineral Acids These are generally prepared from the minerals present in the earth's crust.
e.g., Hydrochloric acid (HCI), sulphuric acid (H2SO4), nitric acid (HNO3), carbonic acid (H2CO3), sulphurous acid (H2SO3), phosphoric acid (H3PO4), etc.
(ii):- Organic Acids or Edible Acids These are produced by plants or animals.
e.g., acetic acid (Source–vinegar), citric acid (Source–lemon, orange), maleic acid (Source–apple), lactic acid (Source–curd), tartaric acid (Source–tamarind), oxalic acid (Source–tomato), methanoic acid (Source–ant sting, nettle sting), etc.
On the Basis of Ionisation
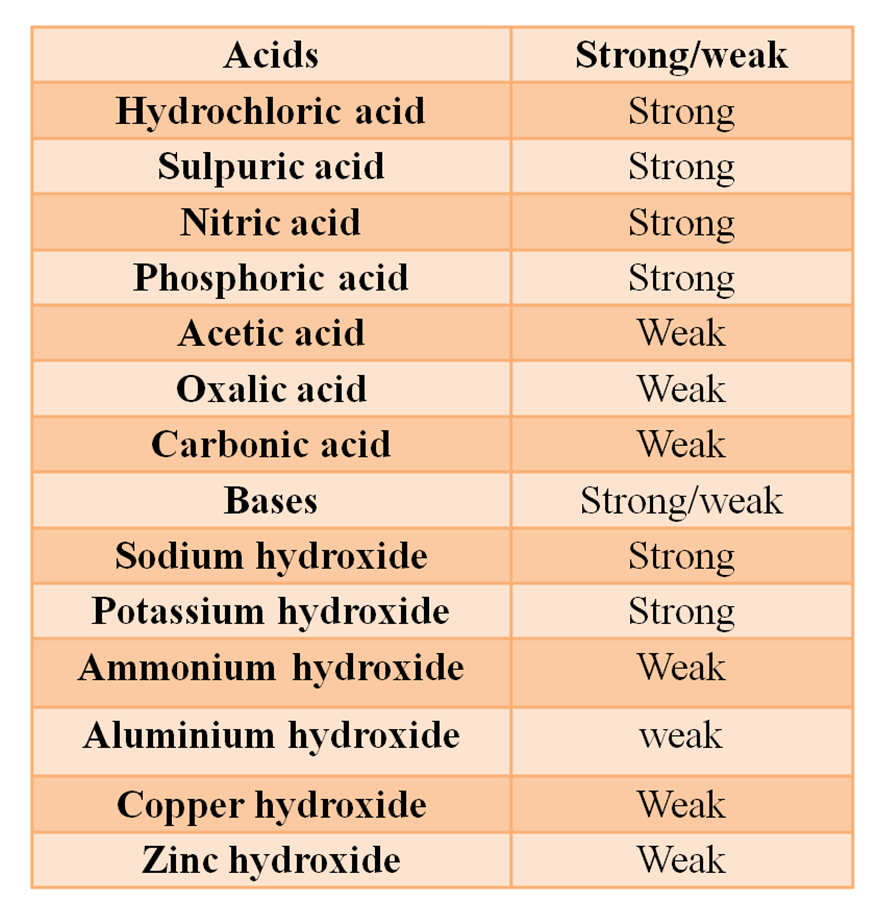
(i):- Strong Acid The acids which ionize almost completely are called strong acids. e.g., mineral acids (except H2CO3).
(ii):- Weak Acid The acids which ionize only partially or to a lesser extent are called weak acids. e.g., organic acids.
On the Basis of the Amount/Concentration of Acid present in Aqueous Solution
(i):- Dilute Acid If in an aqueous solution. Concentration of acid is low, it is called dilute acid.
(ii):- Concentrated Acid If concentration of acid is high, it is called concentrated.
Bases
Substance that furnish hydroxide ions (OH–) in aqueous solution are called bases. Bases are bitter in taste and soapy to touch and turn red litmus solution blue. Their examples are caustic soda or sodium hydroxide NaOH, calcium hydroxide Ca(OH)2, caustic potash or potassium hydroxide KOH, milk of magnesia or magnesium hydroxide Mg(OH)2, etc.
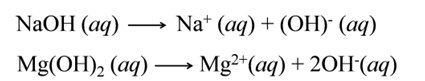
Types of Bases
Bases are of two types:-
(i):- Strong Bases The substances/bases which ionise completely to furnish OH- ions are called strong bases. e.g.,
![]()
(ii):- Weak Bases The bases which ionise only partially are called weak bases. e.g.,
![]()
Alkali
Water soluble base is called alkali. eg., NaOH, KOH, etc. These are usually in laboratory as aqueous solution. These are soapy to touch, bitter and corrosive. Never taste them as they may cause harm. All alkalies are base, but all base, but all base are not alklies.
Strong/Weak Acids and Bases are Tabulated Below
Chemical Properties of Acids
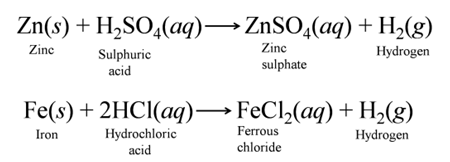
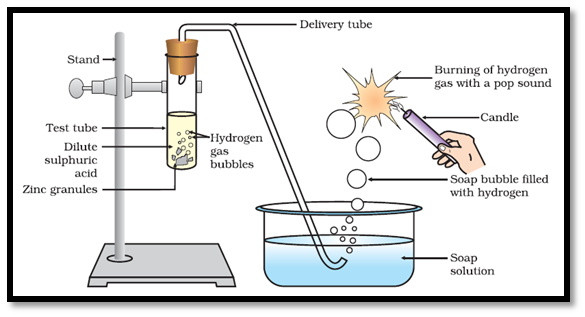
(a) Reaction with Metals Dilute acids (e.g., HCl and H2SO4 not HNO3) react with certain active metals like zinc (Zn), iron (Fe), etc to evolve H2, gas. For Example:-
Test for H2 Gas When a burning match stick is brought near the hydrogen gas, it burns with pop sound.
Metal Oxides
When metal reacts with O2 and H2O present in atmosphere it produces metal oxides. Metal oxides are base in nature.
(b) Reaction with Metal Oxides Acids react with certain metal oxides (basic oxides) to form salt and water. For Example:-
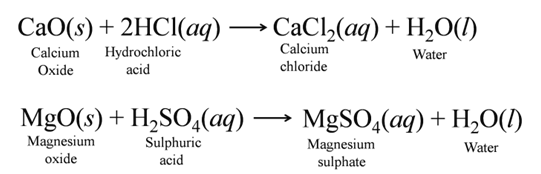
(c) Reaction with Metal Carbonate and Hydrogen Carbonate Acids react with metal carbonates and hydrogen carbonates to produce carbon dioxide gas. 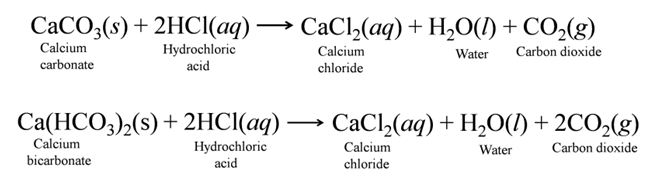
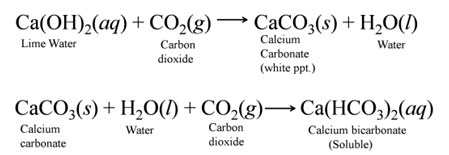
Test for , Gas When gas is passed through lime water, it turns milky due to the formation of white ppt. of CaCO3. But if CO2 is passed in excess, milkiness disappears due to the formation of CaCH(O3)2 which is soluble in water. For Example:-
Chemical Properties of Bases
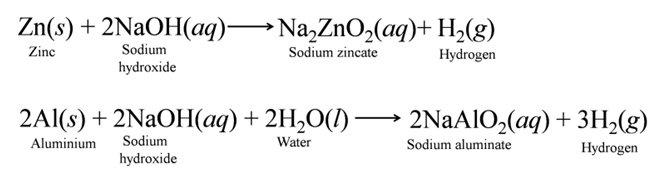
(a) Reaction with Metals Strong bases react with active metals to produce hydrogen gas.

(b) Reaction with Non-metallic Oxide Bases react with non-metallic oxides (acidic oxides) to produce salt and water. This reaction proves that non-metallic oxides are acidic in nature.
Reaction Between Acids and Bases
Acids react with bases to produce salt and water. In this reaction, acid neutralize a base and the reaction is called neutralization reaction. i.e., reduce its effect or vice-versa, thus the reaction is known as neutralisation reaction.

Common Characteristics of Acids and Bases
Both acids and bases conduct free electric current in their aqueous solutions due to the presence of free ions.
Effect of Dilution on an Acid or Base
Mixing of an acid or base with water is called dilution. It results in decrease in the concentration of ions (H3O+/OH–) per unit volume and the acid or base is said to be diluted.
When water is added to an acid or base, their molecules dissociate to form ions. The H+ ions
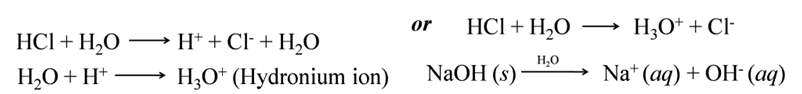
Note:- The process of dissolving an acid or base in water is a highly exothermic reaction. If water is added to a concentrated acid, the heat generated may cause the mixture to splash out and cause burns. To avoid an accident, acid (or base) should be slowly added to the container containing water along with its sides with constant stirring.
Uses of Acids
- Vinegar (acetic acid) is used to preserve pickles and Chinese food.
- In stomach, hydrochloric acid is released which makes the medium acidic and leads to coagulation of proteins. It also helps in digestion and kill the bacteria coming to the stomach along with the food.
- Cold drinks contain carbonic acid.
- Oxalic acid is used to remove ink or rust stains.
- Boric acid is used as an antiseptic and for eye wash.
- Sodium benzoate is used as a preservative in cold drinks.
- Oranges and amla contain ascorbic acid (vitamin C) which prevents scurvy.

 Kaysons Publication
Kaysons Publication
