- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Isomerism
Carbon compounds or organic compounds with same molecular formula can show different structures and hence, different properties. This phenomenon is called isomerism and compounds are called isomers.
For example
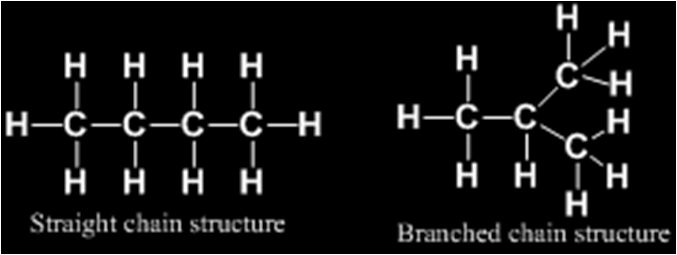
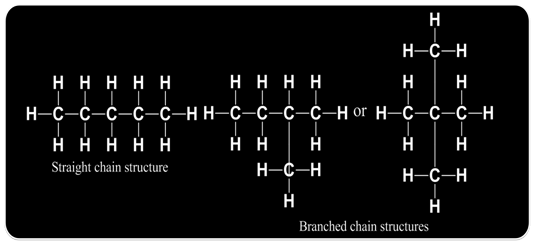
Such pair of isomers is called chain isomers and the isomerism is called chain isomerism.
Molecular formula C5H12
Functional Groups
Functional groups may be defined as an 'atom' or a ‘group of atoms’ which makes a carbon compound reactive and decide its properties regardless of the length and nature of carbon chain.
Homologous Series
A series of similarly constituted compounds in which the members present have the same functional group and similar chemical Properties and any two successive members in a particular series differ in their molecular formula by —CH2— unit, is called a homologous series.
Characteristics of a Homologous Series
- All the members of a homologous series can be represented by the same general formulae.
- Any two adjacent homologues differ by 1 carbon atom and 2 hydrogen atoms in their molecular formulae.
- All the Compounds of a homologous series show similar chemical properties.
- With increase in the molecular mass the gradual change in the physical properties occurs.
- The 14 u is the difference in the molecular masses of any two adjacent homologues:
For example,
CH4, C2H6, C3H8, C4H10 — these all compounds differ by a — CH2 — unit.
Nomenclature of Carbon Compounds
Organic compounds have generally two types of names. These are IUPAC names and common names. The IUPAC names have been adopted by the International Union of Pure and Applied Chemistry. And are based on certain rules. The common names, also known as trivial names, have no proper system for naming.
In general, the IUPAC names of organic compounds are based on the name of basic carbon chain modified by a prefix (phrase before) or suffix (phrase after) showing the name of the functional group.
Writing IUPAC Name of a Compound
Following steps are used to write the name of an organic compound
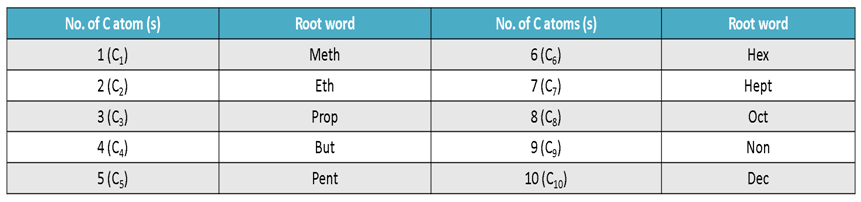
Step I:- Count the number of carbon atoms in the given compound and write the root word for it.
Step II:- If the compounds is saturated, add suffix ‘ane’ to the root word, but if is unsaturated, add suffix ‘ene’ and ‘yne’ for double and triple bonds respectively.
Step III:- If functional group is present in the compounds, it is indicated by adding its suffix.
Root Words for Carbon Atoms

 Kaysons Publication
Kaysons Publication
