- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Allotropes of Carbon and Their Properties
Allotropy is the property by virtue of which an element exists in more than one form and each form has different physical properties but identical chemical properties. These different forms are called allotropes.
Carbon exists in two differents allotropic forms
- Crystalline form
e.g. Diamond, graphite and fullerene.
- Non-crystalline form or amorphous form
e.g. Coal, larrrginriark and charcoal.
Diamond General Properties
- It is a colourless transparent substance with extraordinary brilliance due to its high refractive index.
- It is quite heavy.
- It is extremely hard (hardest natural substance known).
- It does not conduct electricity (because of the absence of free electrons).
- It has high thermal conductivity and high melting point.
- It burns on strong heating to form carbon dioxide.
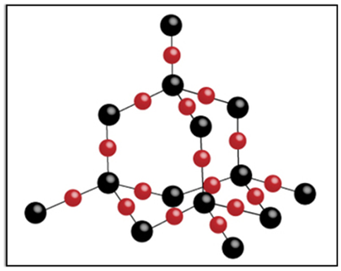
Structure
It is a giant molecule of carbon atoms in which each carbon atom is linked to four other carbon atoms by strong covalent bonds forming a rigid three-dimensional network structure, which is responsible for its hardness.
Uses
- Due to its hardness it is used in knives for cutting marble, granite and glass.
- It is used for the purpose of ornaments studded as precious stones.
- It is used as an abrasive and for polishing hard surface.
- Dies made from diamond are used for drawing wires from the metals.
Graphite General Properties
- It is a grayish black opaque substance.
- It is lighter than diamond, feels soft and slippery to touch.
- Is a good conductor of electricity (due to the presence of free electrons) but bad conductor of heat?
- On strong heating, it burns to give carbon dioxide.
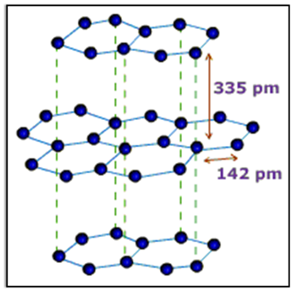
Structure
A graphite crystal consists of layers of carbon atoms or sheets of carbon atoms. Each carbon atom in a graphite layer is joined to three other carbon atoms by strong covalent bonds to form flat hexagonal rings. The various layers of carbon atoms.
Uses
- Is used as a powdered lubricant for the parts of machinery.
- It is used for making electrodes of cells.
- It is used for making lead for pencils as it can mark paper black. It is therefore called black lead or plumbago.
- It can withstand high temperature so it is used for making crucibles to melt substances with high melting points and tiles on the nose cone of space shuttle.
Fullerenes
These are recently discovered allotropic forms of carbon which were prepared for the first time by H W Kroto, Smalley and Robert Curt by the action of laser beam on the vapours of graphite.
General Properties
- These are dark solids at room temperature.
- These are neither too hard nor too soft.
- These are the purest allotropic forms of carbon because of the absence of free valencies or surface bonds.
- On burning, these produce only carbon dioxide gas. Other properties of fullerene are still being investigated.
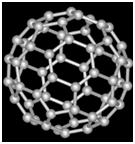
Structure
Fullerene (C60) is a football shaped spherical molecule in which 60 C atoms are arranged in interlocking hexagonal and pentagonal rings of carbon atoms.
Uses
- In pure form, these behave as insulators. However, these can be converted into semiconductors under suitable conditions.
- C60O, a molecule formed when C60 traps O atoms, is used in cancer as well as AIDS therapy.
- In small amounts, these are used to catalyses the photochemical refining in industry.
Versatile Nature of Carbon
The estimated number of carbon compounds known today is about three million.
The main reasons for this huge number of carbon compounds are
Catenation
The property of self linking of elements mainly carbon atoms through covalent bonds to form long, straight or branched chains and rings of different sizes is called catenation.
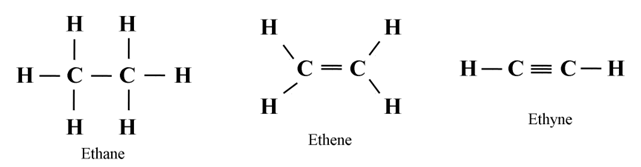
Tetravalency of Carbon
Carbon has four electrons in the outermost shell. Hence, its valency is four i.e. it is capable of bonding or pairing with four other carbon atoms or with the atoms of some other monovalent elements like hydrogen, halogen (chlorine, bromine) etc.
Tendency to form Multiple bonds
Due to its small size carbon has a strong tendency to form multiple bonds (double and triple bonds) by sharing more than one electron pair with its own atoms or with the atoms of elements like oxygen, nitrogen, sulphur etc.

 Kaysons Publication
Kaysons Publication
