- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Classification of Matter
- On the basis of its physical state, such as solid, liquid or gas.
- On the basis of its chemical composition, such as an element compound or mixture.
Matter
- Solid
- Liquid
- Gas
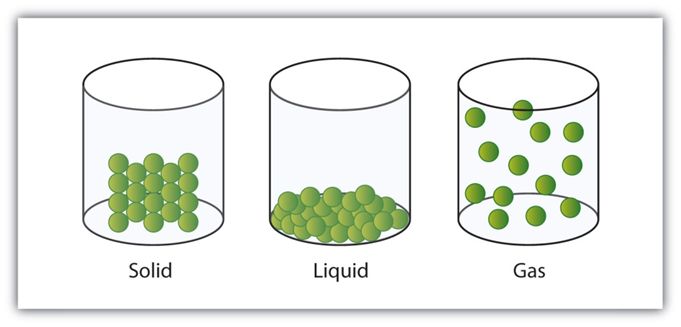
Properties of Solids
- Solids have a fixed shapes and a fixed volume.
- Solids cannot be compressed much.
- Solids have high densities. They are heavy.
- Solids do not fill their container completely.
- Solids do not flow.
Properties of Liquids
- Liquids have a fixed volume but they have no fixed shape.
- Like solids, liquids cannot be compressed much.
- Liquids have moderate to high densities.
- Liquids can take the shape of container in which they kept..
- Liquids generally flow easily.

Properties of Gasses
- Gases have neither a fixed shape nor a fixed volume.
- Gases can be compressed easily
- Gases have very low densities.
- Gases fill their container completely.
- Gases flow easily.

Why Solids, Liquids and Gases Have Different Properties
According to the kinetic theory of matter, the particles of matter are in continuous motion.
Some force of attraction tends to hold the particles together and control their movements.
To Show that Liquids do not have a Fixed Shape but they have a Fixed Volume
Does Gases have fixed shape and fixed volume why?
Why Solids and Liquids Cannot be Compressed but Gasses Can be Compressed Easily?
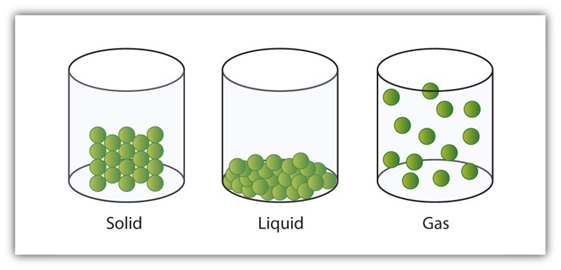
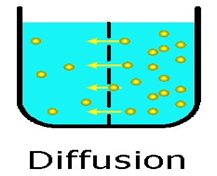
Diffusion
Diffusion is a property of matter which is based on the motion of its particles. Diffusion is fastest in gases and slowest in solids. The rate of diffusion increases on increasing the temperature.
The Common Unit of Temperature and SI Unit of Temperature
We use Celsius scale of temperature for measuring temperatures in our everyday life.
The laboratory thermometers which we use for performing science experiments the clinical thermometer which we use for measuring human body temperature
K = oC + 273
OoC = 273K

 Kaysons Publication
Kaysons Publication
