- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Newlands' Law of Octaves
In 1866. John Newlands, an English scientist; arranged the kown elements in the order of increasing atomic masses' and found that every eighth element had properties similar to that of the first. He called it the law of octaves. This is known as Newland’s law of octaves.
For Example
Newlands’ Octaves
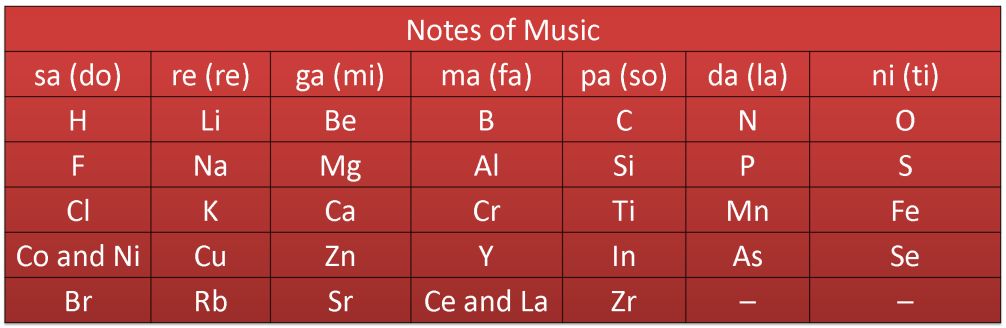
Application
It clearly represented that there was some systematic relationship between the atomic masses and repetition of properties of elements.
Limitations
- This law was successful only up to calcium. After that every eight element did not possess the same properties as those possessed by the elements lying above it in the same group.
- Newlands thought that there were only 56 elements that existed in nature and no more elements would be discovered in the future.
- In order to fit clement into his table, Newlands' adjusted two elements in the same slot, and also put some unlike elements under the same slot.
For Example, cobalt and nickel placed in the same column as fluorine, chlorine and bromine which have very different properties then these elements Iron, which resembles cobalt and nickel in properties, had been placed far away from these elements.
Mendeleev's Periodic Table (1834 – 1907)
Dmitri Ivanovich Mendeleev, a Russian chemist, was the most important contributor for the development of periodic table. 63 elements were known at the Mendeleev's time.
To classify elements
- He examined the relationship between the atomic masses of the elements and their physical and chemical properties.
- Among chemical properties, he concentrated on the compounds formed by the elements with oxygen and hydrogen.
- He took the formulae of the hydrides and oxides formed by an element as one of the basic properties.
Mendeleev's Periodic Law
On this basis Mendeleev formulated a periodic law, which states, “the physical and chemical properties of elements are the periodic function of their atomic masses.”
Features of Mendeleev's Periodic Table
The important features of this table are
- This table contains 8 vertical columns, called groups and 6 horizontal rows, called periods.
- It contains gaps for the elements not discovered at that time. He named such elements by prefixing a Sanskrit numeral eka (one), divi (two), tri (three), etc, to the name of the preceding similar (analogous) clement in the Same group.
For Example eka-boron, eka-silicon, which after their discovery were named as scandium, gallium and getmanium respectively.
- He also predicted the atomic masses and properties of several elements that were not known at that time.
- In order to group the elements having similar properties together, at some places. Mendeleev had to place an element with a slightly greater atomic mass before an element with a slightly lower atomic mass.
- With the help of this table, Mendeleev corrected atomic masses of some elements.
For Example, he corrected the atomic mass of be from 13 to 9.
Limitations of Mendeleev's Periodic Table
This table was greatly helpful for the study of elements but a few anomalies could not be explained on the basis of the table.
These anomalies are
- Position of Hydrogen
- Position of Isotopes
- Uncertainty in Atomic Masses
- Placing of Heavier Element before the Lighter One
Position of Hydrogen
In outer electronic configuration, hydrogen resembles with alkali metals, but tuft like halogen it exists in diatomic form and combines with metals and non-metals to form covalent compounds. Thus, its position was not fixed. But it was kept with alkali metals.
Position of Isotopes
Isotopes are the elements, having similar chemical properties but different atomic masses. In Mendeleev's periodic table, no place was given to these elements"
Uncertainty in Atomic Masses
Another problem was that the atomic mass do not increase in a regular manner in going from one, element to the next. So, it was not possible to predict how many elements could be discovered between two elements especially when we consider the heavier elements.
Placing of Heavier Element before the Lighter One
Few elements, those possess higher atomic mass were placed before elements having a lower atomic mass.
For Example, Argon (39.9) was placed before potassium 39.1, Cobalt (58.9) before nickel (58.6), tellurium (127.60) before iodine (126.9).

 Kaysons Publication
Kaysons Publication
