- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Organic Compounds
The compounds of carbon except its oxides, carbonates and hydrogen carbonate salts, are known as organic compounds. These compounds were initially extracted from natural substances.
Hydrocarbons
The organic compounds containing only carbon and hydrogen are called hydrocarbons.
The hydrocarbons can be classified as
- Saturated hydrocarbons
- Unsaturated hydrocarbons
Saturated Hydrocarbons
The hydrocarbons in which all the carbon atoms are connected by only single bonds are called saturated hydrocarbons or alkanes.
The general formula of these compounds is CnH2n + 2
Unsaturated Hydrocarbons
Those carbon compounds in which at least one double or triple bond is present are called unsaturated compounds.
Unsaturated compounds are further divided into following two classes
- Alkenes (CnH2n)
- Alkynes (CnH2n – 2)
Alkenes
Those carbon compounds which have at least one double bond along with single bonds are called alkenes.
General formula of these compounds is CnH2n.
Alkynes
Those unsaturated hydrocarbons which have one or more triple bonds along with the single bonds are called alkynes.
General formula of these compounds is CnH2n – 2.
How to Draw the Structure of Saturated and Unsaturated Compound?
To draw the structure of carbon compound
Step I:- First connect all the carbon atoms by a single bond.
Step II:- After that satisfy the tetravalency of carbon with available hydrogen atom.
Step III:- If number of available H atoms are less than what is required, satisfy the remaining valency by using double or triple bond in between C atoms.
Structure of Ethane (C2H6)
![]()
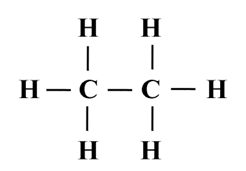
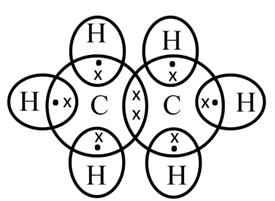
Electron dot structure of ethane (C2H6)
Structure of Propane (C3H8)
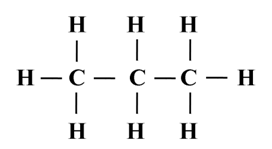
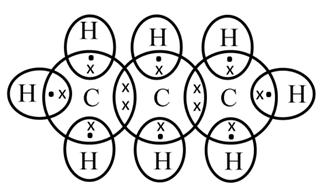
Electron dot structure of propane (C3H8)
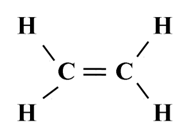
Structure of Ethene (C2H4)
![]()
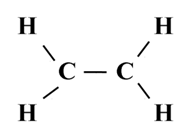
To satisfy it, a double bond is used between the two carbon atoms.
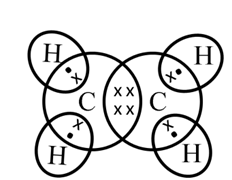
Electron dot structure of ethane
Structure of Ethyne (C2H2)
C ─ C
H ─ C ≡ C ─ H
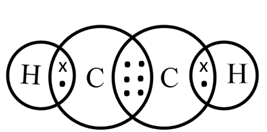
In ethyne, the two carbon atoms share three pairs of electrons among themselves to form a carbon-carbon triple bond.
Electron dot structure of ethyne (C2H2)
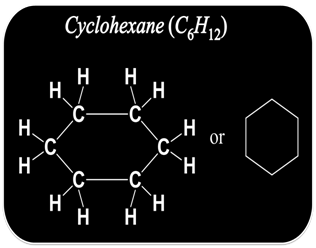
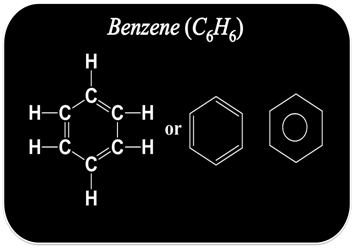
How to Draw Structure of Cyclic Compound?
Some carbon structure also exist in cyclic or ring structure.
To draw the structure of cyclic or ring compound
Step I:- First connect the available carbon atoms by a single bond in the cyclic form.
Step II:- Try to satisfy the tetra valency of each carbon with the available hydrogen atoms.
Step III:- Now check the valency of each carbon. If it is found unsatisfied, use double or triple bond to satisfy it.
For example

 Kaysons Publication
Kaysons Publication
