- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 3
Metals and Non-metals
Introduction
About 118 chemical elements are known at present. On the basis of their properties, all the elements can be divided into two main groups i.e., metals and non-metals. Apart from metals and non-metals, some elements show properties of both metals and non-metals. These are called metalloids.
Both metals and non-metals are very important in our everyday life. We also use a large number of compounds of metals and non-metals.
Metals
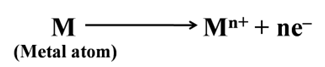
Elements that are electropositive in nature are called metals. They have a tendency to lose electrons and form positive ions. For example, copper, iron, aluminium, sodium, etc.

Physical Properties of Metals
- Malleability It is the property of metal due to which it can be beaten into thin sheets.
- Ductility It is the property due to which a metal can be drawn into wires.
- Hardness Most of the metals are hard. But some alkali metals like sodium and potassium are so soft that they can be cut easily with knife.
- Metallic Lustre Metals in their pure state have bright shining surfaces.
- Electrical Conductivity Most of the metals are good conductors of electricity.
- Thermal Conductivity Generally metals are good conductors of heat, except lead and mercury.
- Melting and Boiling Points Metals generally have high melting and boiling point, except mercury, gallium and alkali metals.
- Sonority When metals are struck with a hard substance, they produce sound.
Chemical Properties of Metals
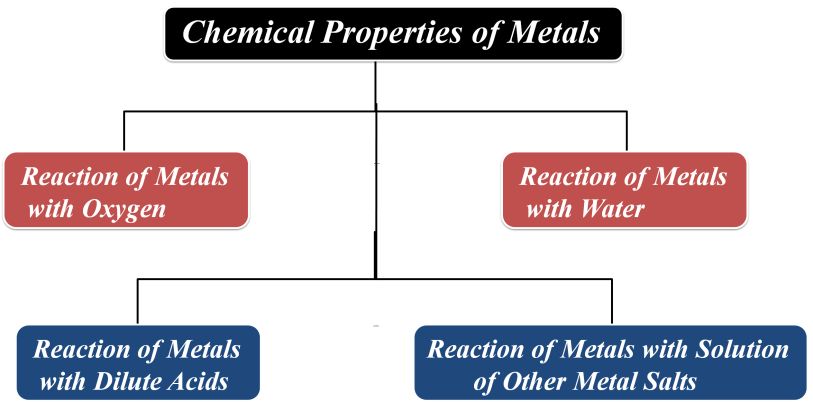
Most of the chemical properties of metals are due to their electropositive nature. It means that a metal atom lose electrons to form cations.
Reaction of Metals with Oxygen
Burning in Air (Formation of oxides)
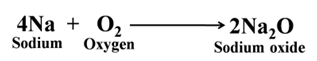
Almost all the metals react with oxygen (or air) to form metal oxides.
![]()
For example, when copper is heated in air, it combines with oxygen to form copper (II) oxide, a black oxide.
![]()
Similarly, sodium forms sodium oxide
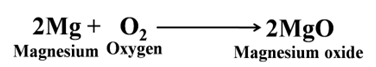
Magnesium forms magnesium oxide
Aluminium forms aluminium oxide
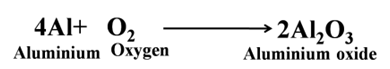
Nature of Metallic Oxides
(Formation of hydroxides)
Most of the metallic oxides are basic in nature. When dissolved in water, they produce alkali. For Example:-
Similarly, sodium oxide forms sodium hydroxide
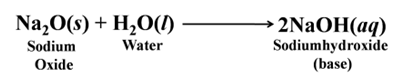
Note:- Alkalis are the bases that dissolve in water.
Exception
Some metal oxides show both types of behavior, i.e., acidic and basic. These oxides are called amphoteric oxides. e.g., zinc oxide, aluminium oxide, etc. For Example:-
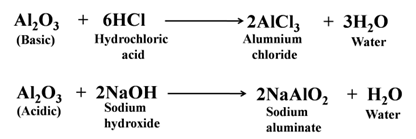
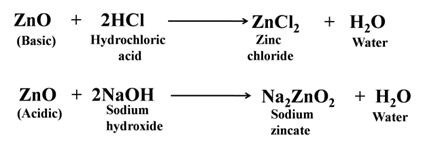
Order of Reactivity with Oxygen
Different metals react with oxygen at different rates. For example, sodium (Na) and potassium (K) catch fire, when placed in the open. Hence, these are most reactive metals. To prevent accidental fires, these metals are kept immersed in kerosene oil. Magnesium (Mg) burns in air only by heating. Zinc (Zn) burns only on strong heating while iron does not burn in the form of rod or block but burns in the form of filings. Copper (Cu) does not burn on heating but blister copper burns.
Oxide silver does not react with oxygen. Hence, the order of reactivity of these metals with oxygen is Na > Mg > Zn > Fe > Cu > Ag.
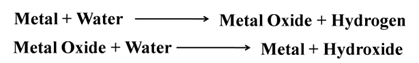
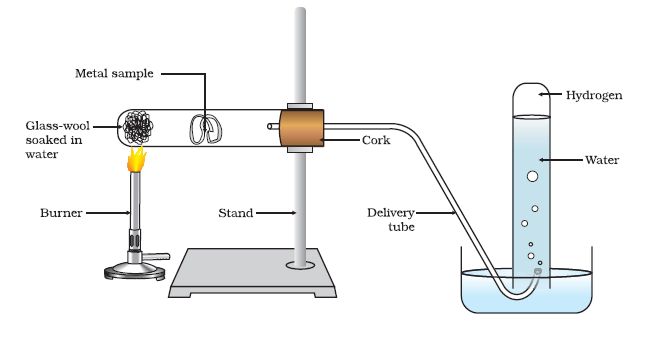
Reaction of Metals with Water
Metals react with water and produce a metal oxide and hydrogen gas. Metals oxides that are soluble in water dissolve into further to form metal hydroxides but all metals do not react with water.
Metals like potassium and sodium react violently with cold water. In case of Na and K, the reaction is very violent and exothermic.
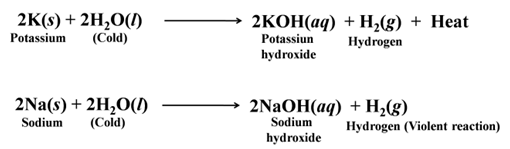
The, heat evolved is sufficient for hydrogen to catch fire. That’s why Na and K catch fire when kept in water.
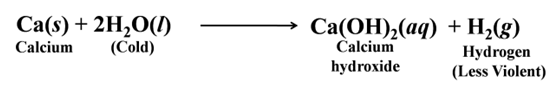
The reaction of calcium with water is less violent. The heat evolved is not sufficient for the hydrogen to catch fire.
Magnesium does not react with cold water. It reacts with hot water and forms magnesium hydroxide and hydrogen.
It also starts floating due to the bubbles of hydrogen gas sticking to its surface.
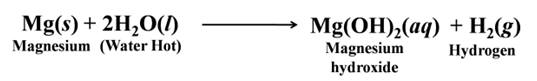
Metals like aluminium, iron and zinc do not react either with cold or hot water. They react with steam and form the metal oxide and hydrogen.
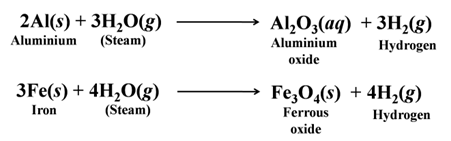
Lead, copper, silver and gold do not react with water at all. Thus, the reactivity order of metals towards water is
K > Na > Ca > Mg > Al > Fe > Pb > Cu > Ag > Au.
Reaction of Metals with Dilute Acids
- Reaction of Metals with Dilute HCl and Dilute H2SO4.
Except a few less reactive metals (Cu, Hg, Ag, Au, Pt, etc) all metals react with dilute sulphuric acid and hydrochloric acid to produce salt and hydrogen gas. For Example:-
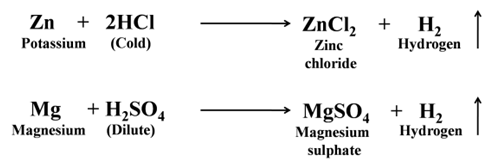
Reaction of Metals with Dilute HNO3 Except magnesium and manganese, metals do not react with dilute nitric acid. This is due to the oxidising nature of nitric acid.
Normal Case:-
![]()
![]()
Exceptional Case (for Mn and Mg only)
Aqua Regia
It is a freshly prepared mixture of concentrated hydrochloric acid and concentrated nitric acid in the ratio of 3 : 1. It can dissolve gold, even though neither of these acids can do so alone.
Aqua regia is a highly corrosive, fuming liquid; It is one of the few reagents that is able to dissolve gold and platinum.
Reaction of Metals with Solution of Other Metal Salts
A reactive metal can displace a comparatively less reactive metal from their compounds in aqueous solution or in molten state.
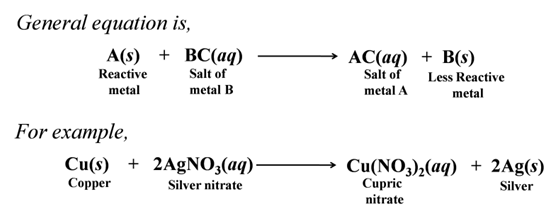
Reactivity Series or Activity Series of Metals
The arrangement of metals in the order of their decreasing reactivities is called reactivity series of metals.
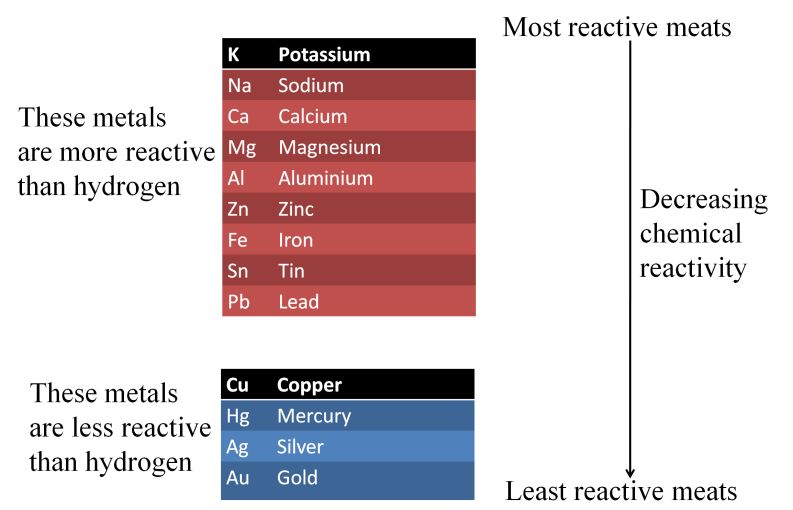
Importance of Reactivity Series
The following two points state importance of reactivity series
- In reactivity series, metals that are placed above hydrogen, can displace hydrogen from dilute acids (HCI and H2SO4).
- Metal placed above another metal in this series can displace it from the solutions of its salt, as the metal towards top is more reactive than a metal placed below it.

 Kaysons Publication
Kaysons Publication
