- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Indicators
Indicators
The easiest way to detect whether a solution is acidic, alkaline or neutral is to use an indicator. Indicators are substances that change their colour or odour if they are put into an acid or alkaline solution. Litmus, turmeric and China rose extract, etc are natural indicators.
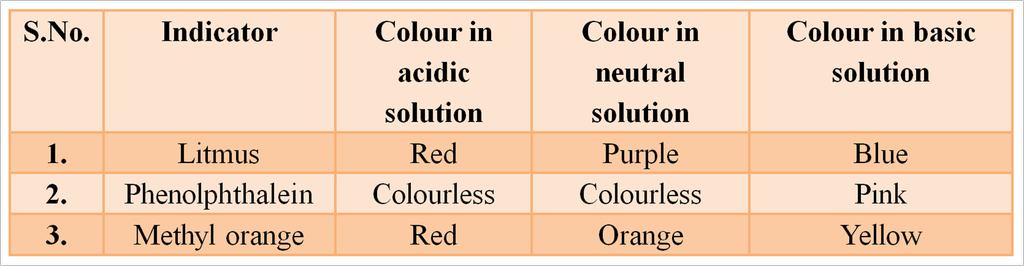
Some Natural Indicators with Characteristic Colour
Synthetic Indicators
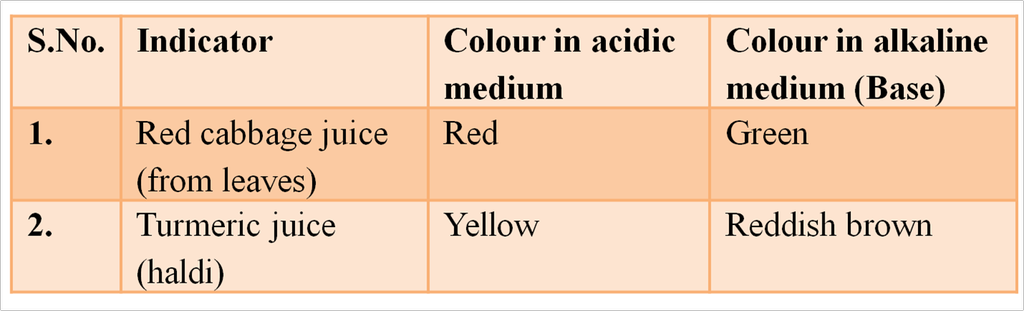
Those chemical substances which change their colour in acids and bases are called synthetic indicators.
They are also called synthetic acid base indicators. e.g., methyl orange, phenolphthalein methylene blue, and methyl red are synthetic indicator.
Some Synthetic Indicators with Characteristic Colour
Olfactory Indicators
Those substances whose odour changes in acidic or basic media are called olfactory indicators.
Vanilla, onion and clove can be used as olfactory indicator.
Litmus Indicators
It is purple dye, which is extracted from lichen, a plant from the division Thallophyta. It is commonly used as acid-base indicator. When the litmus solution is neither acidic nor basic, its colour is purple. When purple solution is acidified, it changes to blue colour whereas it changes to red colour when a small amount of base is added to blue litmus solution.
Strength of an Acid or Base
Strength of an acid or base depends on the number of H+ ions or OH- ions produced by them respectively.
Larger number the of H+ ions produced by an acid, stronger is the acid. Similarly, larger the number the OH- ions produced by a base, stronger is the base.
To judge how strong a given acid or base is, a universal indicator is used which is mixture of several indicators. It shows different colours at different concentrations of hydrogen ion in a solution.
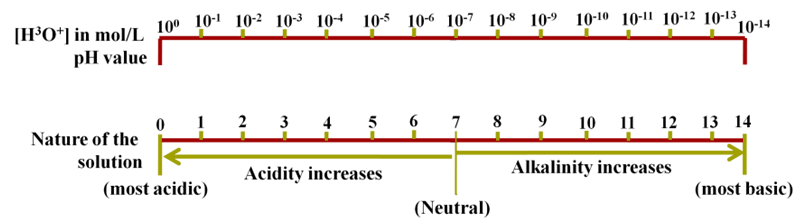
The pH Scale
It is a scale used for measuring hydrogen ion concentration. The p in the pH stands for potenz which means power in German. It has values zero (very acidic) to fourteen (very alkaline).
pH is number which indicates the acidic or basic nature of a solution. Higher the hydronium ion concentration present in the solution lower is their pH value.
{pH means power of hydrogen ions}
- If pH > 7, solution is basic
- If pH < 7, solution is acidic
- If pH = 7, solution is neutral
Importance of pH in Everyday Life
Following are the examples showing importance of pH in everyday life
(i):- pH of Soil Every type of plant requires a specific pH range for healthy growth. Therefore, the nature of soil is known first by testing its pH and then a particular crop is grown in it. It is also suitable for selecting the fertiliser for a particular crop by knowing the pH of he soil.
(ii):- Effect pH on our Digestion HCl present in the stomach helps in the digestion of food. When acidity increases in the stomach, pain, irritation and indigestion is caused. To correct the disturbed pH range, milk of magnesia is used as a medicine, which is also called antacid as it reduces the
effect of acid (or acidity).
(iii):- Effect of pH in Tooth Decay Tooth enamel is made up of calcium phosphate and is the hardest substance in the body. If the pH inside the mouth decreases than 5.5, the decay of tooth enamel begins. This happens when the bacteria present in the mouth work on the left over food particles and produce acid. To prevent tooth decay toothpastes (basic) are used which neutralise the excess acid.
(iv):- When insects like honeybee, ant, etc bite; they leave an acid in the skin, that causes pain and irritation. If a mild base like baking soda is applied on the affected area, it gives relief.
(v):- Stinging Hair of Neetle leaves injects methanoic acid in the skin which causes pain. It is cured by rubbing the affected area with the leaves of dock plant, found in the same locality where the neetle plant is found.

 Kaysons Publication
Kaysons Publication
